Method for preparing febuxostat intermediate
A technology of febuxostat and intermediates, applied in the field of medicinal chemistry, can solve the problems of colloid precipitation, easy adsorption products, brominated isobutane cannot be recovered, and increase the difficulty of separation and purification, so as to avoid high boiling point solvents and toxic The introduction of substrates is beneficial to the control of product-related substances and the effect of reducing the possibility of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
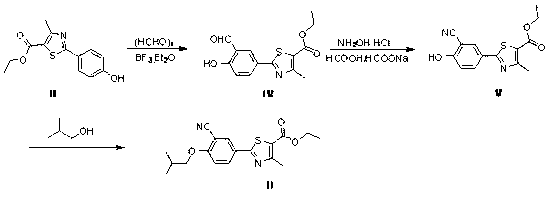
[0049] (1) Preparation of the compound of formula IV:
[0050] First add 0.56 g of boron trifluoride diethyl ether and 40 ml of acetonitrile into the reaction flask, and then add 4.2 g of paraformaldehyde. N 2 protection, finally add 5.26g of the compound of formula III, heat to 70-80°C for reaction, and the reaction time is 8 hours; Between ~6, the product was precipitated by cooling, and filtered to obtain a yellow solid. The dried solid was 5.46g, and the yield was 94%.
[0051] (2) Preparation of the compound of formula V:
[0052] Add 11g of formic acid to the three-neck flask, take 3.8g of the compound of formula IV, 2.8g of hydroxylamine hydrochloride, and 2.8g of sodium formate, heat to 100°C for reaction, and the reaction time is 4 hours; after the reaction, cool down to room temperature and pour into an appropriate amount of ice water , stirred and filtered, the filter cake was washed with purified water, and then dried under reduced pressure at 50°C overnight to ...
Embodiment 2~6
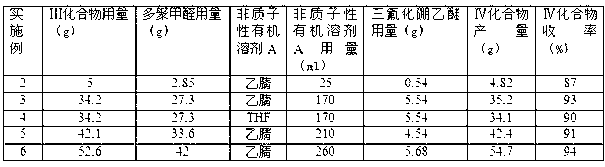
[0057] Tables 1 to 3 have listed the technical characteristics and results of implementing this program under different conditions, and the specific operation method is the same as in Example 1.
[0058] Formylation parameters and results of Table 1 Examples 2-6
[0059]
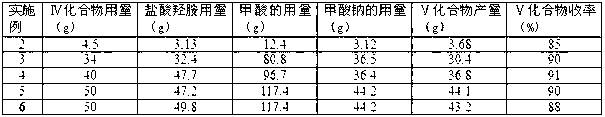
[0060] Table 2 Aldoxime dehydration reaction parameters and results of Examples 2-6
[0061]
[0062] Table 3 Mitsunobu etherification reaction parameters and results of Examples 2-6
[0063] The mp of compound II obtained in the above examples: 174-175°C.
[0064] 1H-NMR(CHCl3)1.084-1.101d,6H,1.374-1.409(dd,3H),2.173-2.223(m,1H),2.764(s,3H),3.892-3.908(d,2H),4.331-4.384 (s, 2H), 7.001-7.023 (d, 1H), 8.074-8.101 (d, 1H), 8.168-8.173 (s, 1H).
[0065] As can be seen from the results of the above examples, the preparation of febuxostat intermediates by the method of the present invention avoids the introduction of high boiling point solvents and toxic catalysts, and has the advantages of simple aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com