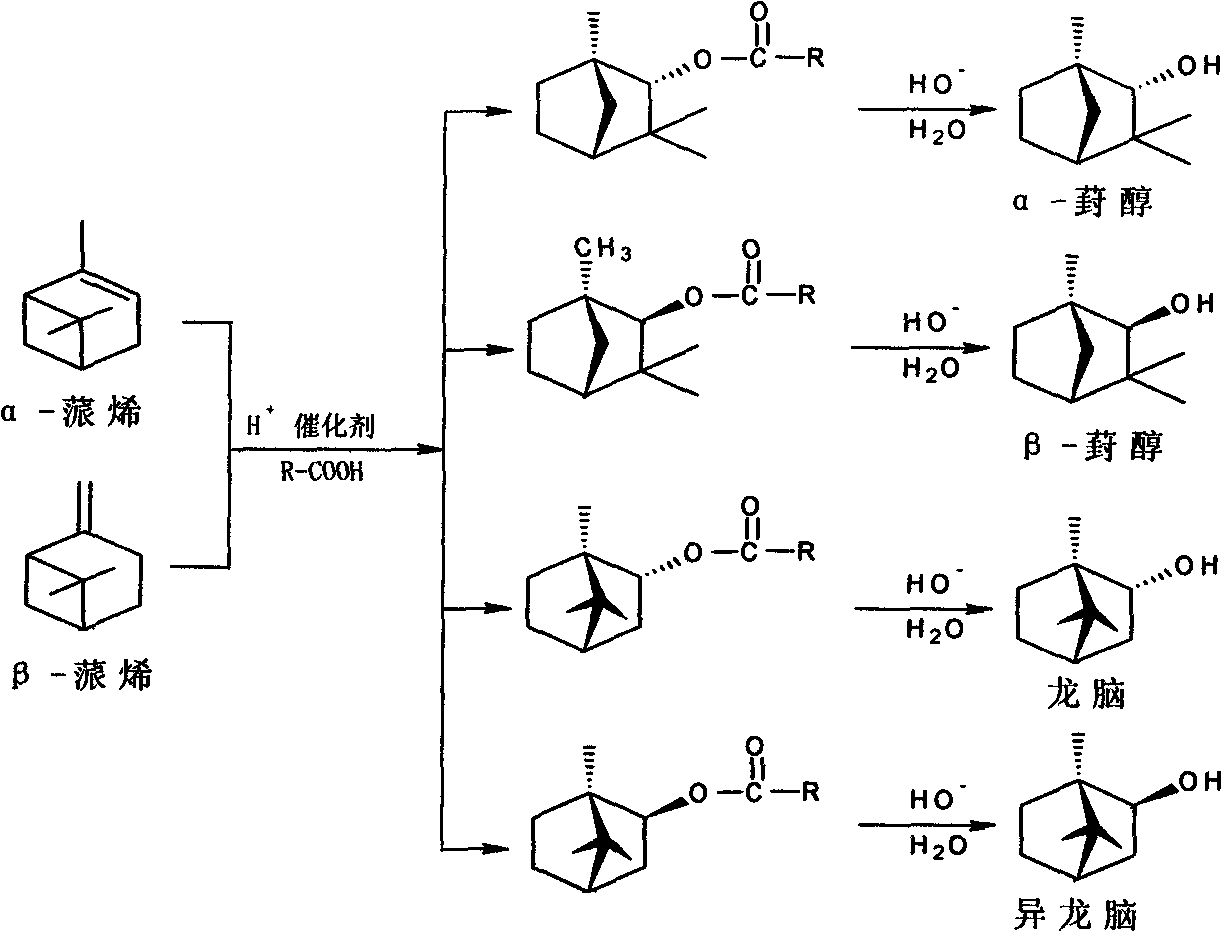
One-step method for synthesizing fenchol with turpentine
A technology for turpentine and fenol, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of oxygenated compounds, etc., can solve the problems of difficulty in obtaining high-purity fenol products, limited sources of raw materials, long process routes, etc. variety, increase fiscal revenue, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
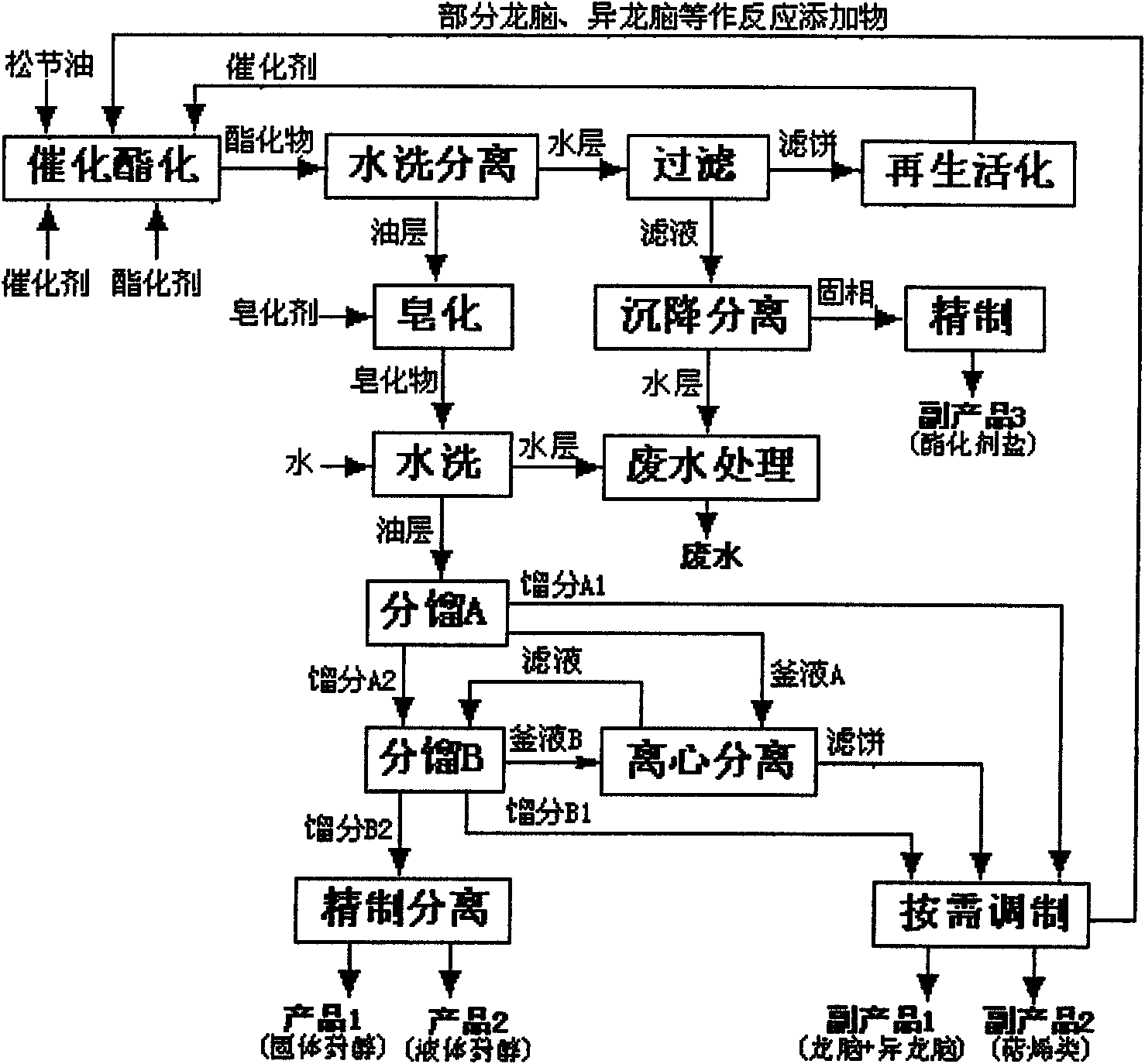
[0041] (1) Process setting:
[0042] Raw material formula (parts by weight): 12 parts of turpentine, 0.48 parts of a catalyst with a 1:1 ratio of inorganic acid and metal oxide, 2.26 parts of an esterifying agent with a 1:0.6 ratio of oxalic acid and oxalic acid, and 0.04 parts of an aromatic hydrocarbon additive.
[0043] Process parameters: Esterification temperature 85°C, esterification time 20h, first washing standing for 18h, saponification temperature 85°C, saponification time 7h, second washing standing 16h, fractionation A top temperature control is less than 100°C, fractionation B top temperature Control 100 ~ 140 ℃, crystallization temperature 2 ℃.
[0044] (2) Preparation method:
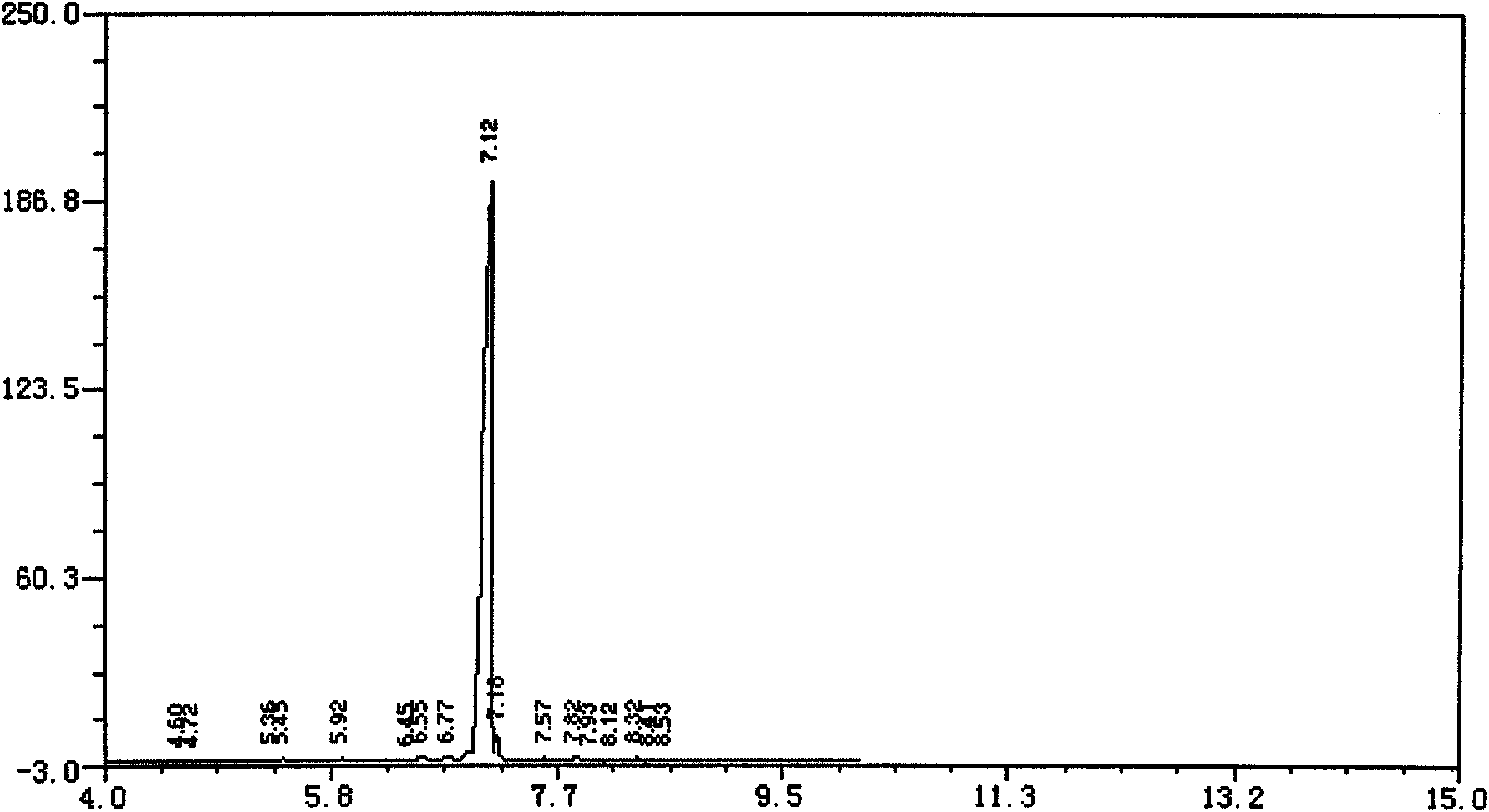
[0045] Add turpentine, catalyst, esterifying agent, and additives into a 20L glass reactor respectively, stir and raise the temperature to 85°C, and keep it for 20h. Sampling was carried out, and the reaction mixture was detected by GC. The pinene content was 0.42% (99.5% conversion rat...
Embodiment 2
[0049] (1) Process setting:
[0050] Raw material formula (parts by weight): 12 parts of turpentine, 0.60 part of catalyst of kaolin and metal oxide ratio of 1:1, 2.4 parts of esterifying agent of oxalic acid, p-toluenesulfonic acid and oxalic acid of 0.8:1:0.3 ratio, aliphatic hydrocarbon 0.06 part of auxiliary agent.
[0051] Process parameters: Esterification temperature 95°C, esterification time 22h, first wash and stand for 20h, saponification temperature 90°C, saponification time 6.5h, second wash stand for 20h, fractionation A top temperature control is less than 100°C, fractionation B top The temperature is controlled at 100-140°C, and the crystallization temperature is 8°C.
[0052] (2) Preparation method:
[0053] Add turpentine, catalyst, esterification agent and auxiliary agent respectively into a 20L glass reactor, stir and raise the temperature to 95°C, and keep it for 22h. Sampling was carried out, and the reaction mixture was detected by GC. The pinene content...
Embodiment 3
[0057] Raw material formula (parts by weight): 200 parts of turpentine, 100 parts of a mixture of by-product borneol, isoborneol and terpene in a ratio of 0.5:0.3:2, 13.9 parts of a catalyst of kaolin, inorganic acid and metal oxide in a ratio of 1:0.5:0.5 , 60 parts of an esterification agent with a ratio of 0.6:1 between oxalic acid and p-toluenesulfonic acid, and 1.2 parts of an auxiliary agent with a ratio of 1:0.5 between aromatic hydrocarbons and aliphatic hydrocarbons.
[0058] Preparation:
[0059] Add turpentine, by-product mixture, catalyst, esterification agent, and additives into a 500L enamel reaction kettle, stir and raise the temperature to 110°C, and keep it for 20h. Sampling was carried out, and the reaction mixture was detected by GC. The pinene content was 1.04% (98.4% conversion rate), and the reaction was stopped. The esterification reaction product was washed with water and left to stand for 24 hours to separate the oil layer and water layer mixture. Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com