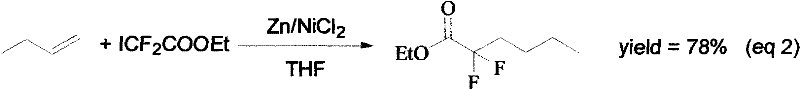
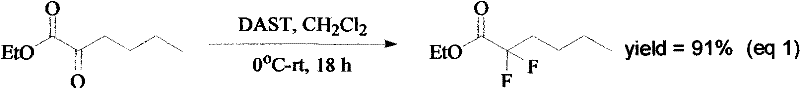Synthesis method of ethyl 2,2-difluorohexanoate
A technology of ethyl difluorohexanoate and dosage, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., can solve the problems of inconvenient synthesis and high price of ethyl iodide difluoroacetate, and achieve production Low cost, high economic value, and convenient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add Zn (0.2mol, 11.2g) and NiCl to a three-necked flask (250mL) 2 .6H 2 O (0.01 mol, 0.167 g) and then THF (80 mL) was added. Add BrCF dropwise at 35℃ 2 COOEt (0.1mol, 20.3g), after reaction for 0.5h, add dropwise a THF solution of butene (0.4mol, 22.4g) at 35°C for 0.5-1.0h. After reacting at 15°C for 5.0h, 19 F NMR monitors the reaction. The reaction solution was poured into a saturated ammonium chloride solution, extracted with ether, washed with water 4 times, dried with sodium sulfate, and evaporated at atmospheric pressure to remove the ether and recover the ether. Pump vacuum distillation to obtain 2,2-difluorohexanoic acid ethyl ester (0.0856mol, 15.4g) with a yield of 85.6%.
Embodiment 2
[0026] Add Zn (0.2mol, 11.2g) and NiCl to a three-necked flask (250mL) 2 .6H 2 O (0.01 mol, 0.167 g) and then THF (100 mL) was added. Add BrCF dropwise at 40℃ 2 COOEt (0.1mol, 20.3g), after reacting for 1.0h, a THF solution of butene (0.4mol, 22.4g) was added dropwise at 40°C for 1.0h. After reacting at 20°C for 5.0h, 19 F NMR monitors the reaction. The reaction solution was poured into a saturated ammonium chloride solution, extracted with ether, washed with water 6 times, dried with sodium sulfate, and evaporated at atmospheric pressure to remove the ether and recover the ether. Pump vacuum distillation to obtain 2,2-difluorohexanoic acid ethyl ester (0.0856mol, 15.8g), the yield was 86%.
Embodiment 3
[0028] Add Zn (0.4mol, 22.4g) and NiCl to a three-necked flask (250mL) 2 .6H 2 O (0.02mol, 0.334g) and then THF (120mL) was added. Add BrCF dropwise at 37℃ 2 COOEt (0.2mol, 40.6g), after reacting for 0.7h, a THF solution of butene (0.4mol, 22.4g) was added dropwise at 38°C for 0.8h. After reacting at 18°C for 5.0h, 19 F NMR monitors the reaction. The reaction solution was poured into a saturated ammonium chloride solution, extracted with ether, washed with water 5 times, dried with sodium sulfate, and evaporated to remove the ether and recover the ether under normal pressure. Water pump vacuum distillation, 2,2-difluorohexanoic acid ethyl ester (0.0816mol, 14.7g) was obtained, and the yield was 81.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com