Glycopyrrolate compounds and synthesis method thereof
A synthesis method and pyrrolose technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of large amount of original drug, high price, no synthesis process, etc., and achieve easy synthesis and solve the shortage of sources. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
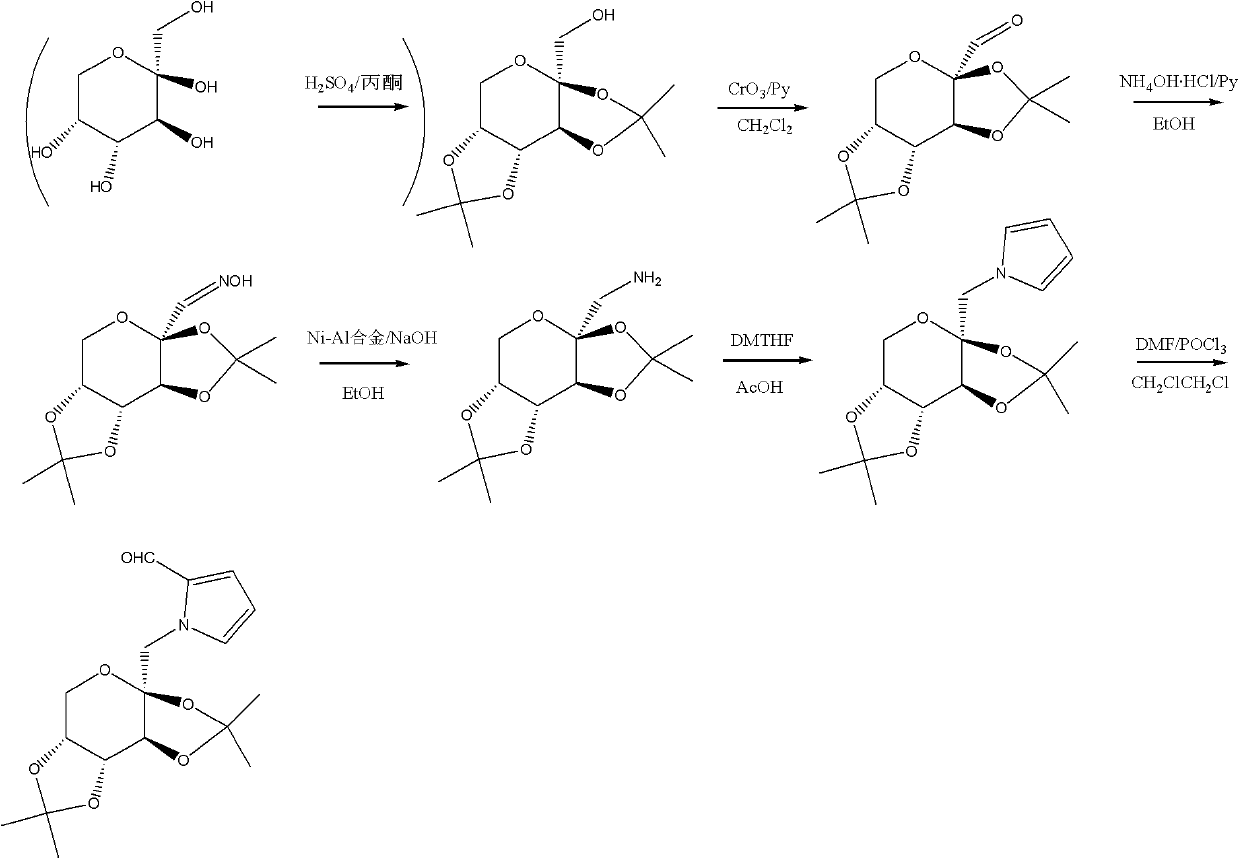
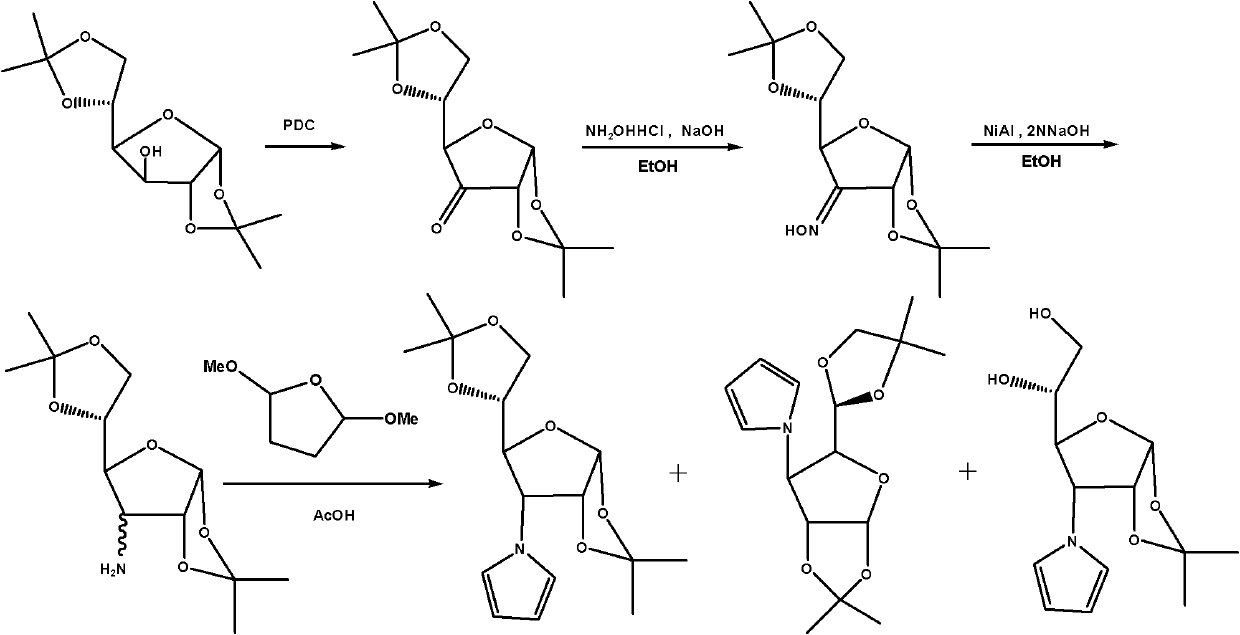
[0031] The present invention further provides a method for synthesizing the above-mentioned pyrrolidose compound, described as method i in this specification, comprising the following steps:
[0032]The other hydroxyl groups except one hydroxyl group of the monosaccharide are protected, and the unprotected hydroxyl group is oxidized to carbonyl group to form an oxime, which is further reduced to an amino compound, and then reacted with 2,5-dimethoxytetrahydrofuran to generate a pyrrolose compound.
[0033] Specifically, the synthetic method i comprises the steps:
[0034] (1) select one of the following methods to carry out hydroxyl protection:
[0035] a. Acetone sulfuric acid introduces isopropylidene protection;
[0036] b. Acetone sulfuric acid, using copper sulfate as a catalyst to introduce isopropylidene protection;
[0037] In above-mentioned steps (1), preferred method a. acetone sulfuric acid introduces isopropylidene protection, namely
[0038] (2) select one of ...
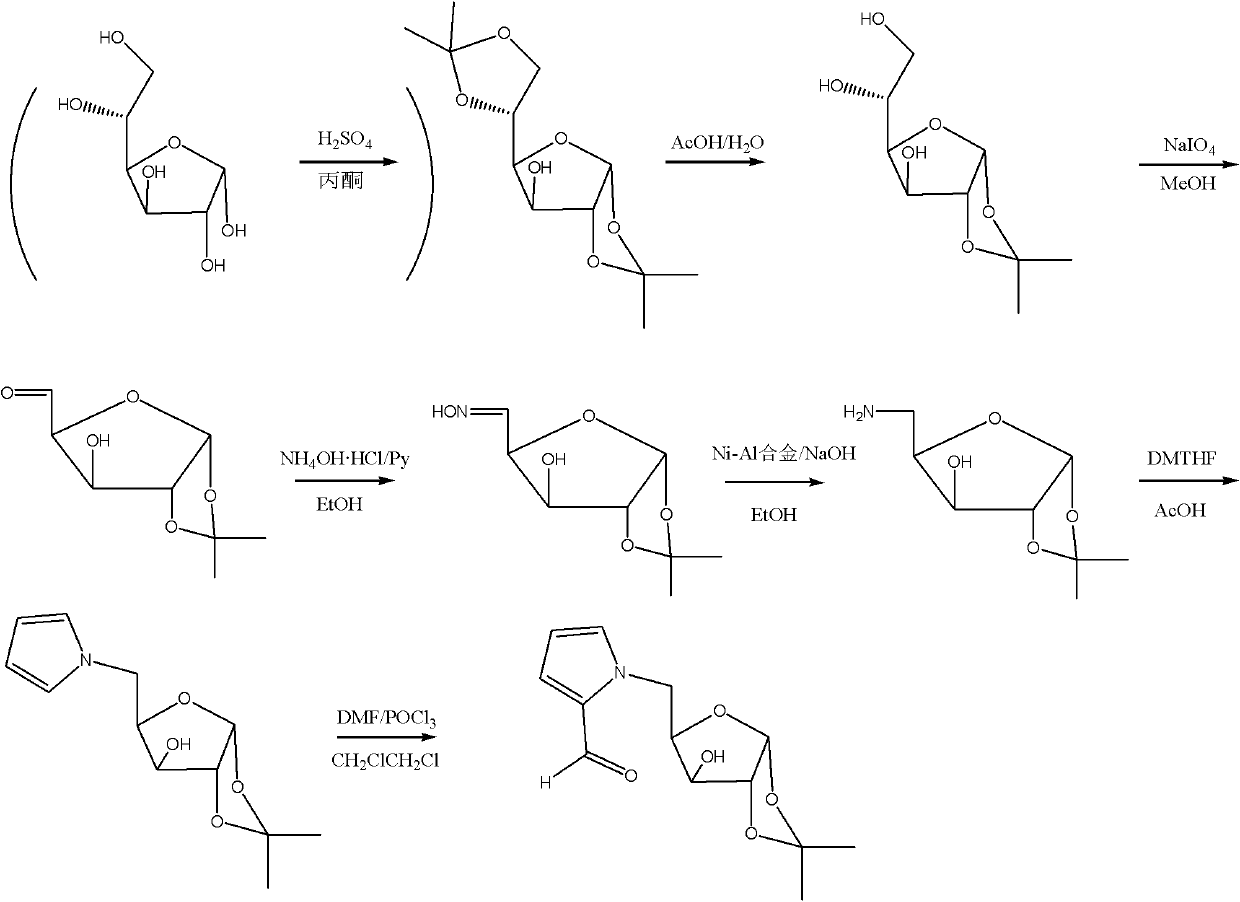
Embodiment 1
[0077] Example 1: Synthesis of 1-pyrrole fructose
[0078] (1) Synthesis of 2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose (1)
[0079]
[0080] Compound 1
[0081] In a 250mL three-necked flask, add 100mL of acetone, 3mL of concentrated sulfuric acid, stir and cool, add 7.5g of D-fructose in batches at 25°C, react at room temperature for 3 hours, neutralize with 1N sodium hydroxide, filter, evaporate the acetone, recrystallize, 6.5 g of white solid were obtained, and the yield was 60.0%.
[0082] (2) Synthesis of 2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranosaldehyde (2)
[0083]
[0084] Compound 2
[0085] Add 300mL CH to the 1000mL three-necked flask 2 Cl 2 , add 35mL pyridine, add 23g CrO 3 . 15.08 g of diacetone fructose (1) was weighed and added to the reaction solution, and then 8.2 mL of acetic anhydride was added, and the reaction temperature was controlled at 20°C-25°C. TLC detected that the reaction was complete, 500 mL of ethyl acetate w...
Embodiment 2
[0103] Example 2: Synthesis of 1-pyrrole fructose by imidazole sulfonate method
[0104] (1) Synthesis of 1-O-imidazolesulfonyl-2,3:4,5-bis-O-(1-methylethylene)-β-D-fructopyranose (7)
[0105]
[0106] Compound 7
[0107] Under nitrogen protection, in a 5000mL three-necked flask, sequentially add 52.0g diacetone fructose, 15.0g imidazole, and 1000mL DMF, cool down to -5°C, add 30g sulfuryl chloride dropwise with stirring, stir for 0.5h, warm up to room temperature and react for 1h, Diethyl ether and ice water were added, the aqueous phase was extracted, the organic phases were combined, washed with brine, dried, evaporated to remove the solvent, and subjected to column chromatography to obtain 62.5 g of solid with a yield of 80.0%. Melting point: 75-76℃, [α] D -27° (c 7.4, MeOH). 1 HNMR (400MHz, CDCl 3 ): δ8.03(s, 1H), 7.36(s, 1H), 7.19(s, 1H), 4.58(dd, J=7.9, 2.6Hz, 1H), 4.25(d, J=10.2Hz, 1H) , 4.21 (dd, J=7.9, 0.7Hz, 1H), 4.18 (d, J=2.6Hz, 1H), 4.16 (d, J=10.2Hz, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com