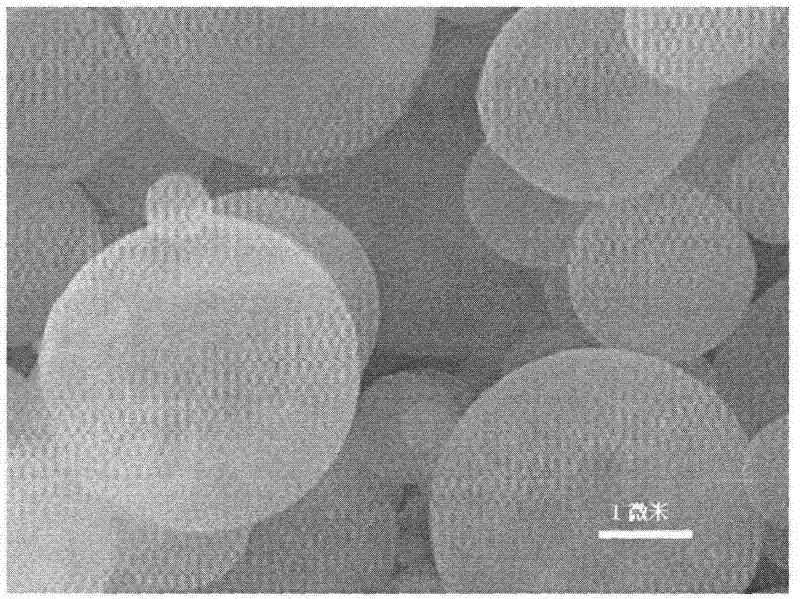
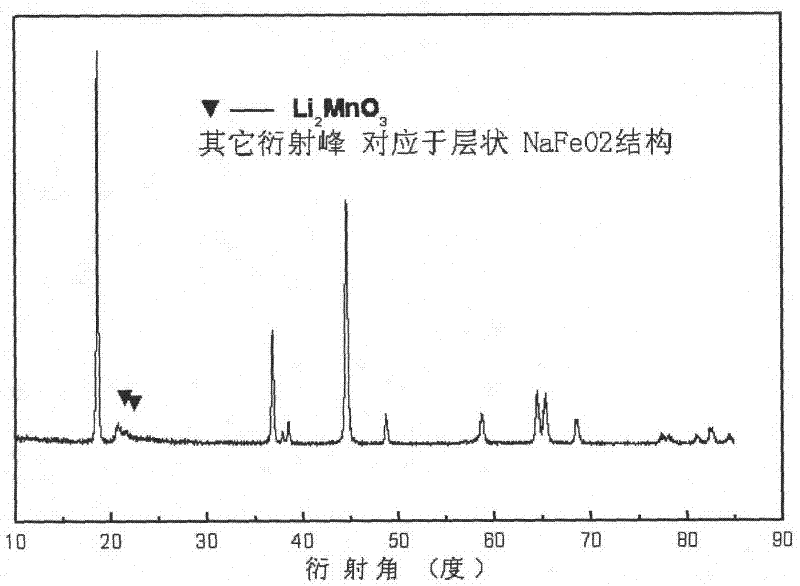
Multiphase Mn (manganese)-base anode material and preparation method thereof
A cathode material, multi-phase manganese-based technology, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of unsteady control of the proportion, low first coulombic efficiency of the battery, affecting the performance of the cathode material, etc. The effect of high content and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The first step: the sulfate salt of nickel and manganese is formulated into a nickel-manganese mixed solution with a total molar concentration of nickel and manganese of 2 mol / L according to the molar ratio of nickel and manganese of 3:7; ammonia water and citric acid are mixed to form a two-component compound Agent solution, wherein the molar concentration of ammoniacal liquor is 0.3mol / L, and the molar concentration of citric acid is 0.4 times of ammoniacal liquor molar concentration; The sodium hydroxide solution that is 2mol / L is used as precipitation agent with concentration. The above-mentioned nickel-manganese mixed solution, compounding agent solution, and precipitant are continuously added to the reactor respectively, the total amount of nickel-manganese mixed solution added is 10 liters, and the total amount of compounded agent solution added is based on the total molar amount of solute in the compounded agent solution Calculated as 0.2 times the total molar am...
Embodiment 2
[0039] The first step: the sulfate salt of nickel and manganese is formulated into a nickel-manganese mixed solution with a total molar concentration of nickel and manganese of 2 mol / L according to the molar ratio of nickel and manganese of 3:7; ammonia water and citric acid are mixed to form a two-component compound Agent solution, wherein the molar concentration of ammoniacal liquor is 0.4mol / L, and the molar concentration of citric acid is 0.2 times of ammoniacal liquor molar concentration; The sodium hydroxide solution that is 2mol / L is used as precipitation agent with concentration. The above-mentioned nickel-manganese mixed solution, compounding agent solution, and precipitant are continuously added to the reactor respectively, the total amount of nickel-manganese mixed solution added is 10 liters, and the total amount of compounded agent solution added is based on the total molar amount of solute in the compounded agent solution Calculated as 0.2 times the total molar am...
Embodiment 3
[0045] The first step: the sulfate salt of nickel and manganese is formulated into a nickel-manganese mixed solution with a total molar concentration of nickel and manganese of 2 mol / L according to the molar ratio of nickel and manganese of 3:7; ammonia water and citric acid are mixed to form a two-component compound Agent solution, wherein the molar concentration of ammoniacal liquor is 0.6mol / L, and the molar concentration of citric acid is 0.5 times of ammoniacal liquor molar concentration; The sodium hydroxide solution that is 2mol / L is used as precipitation agent with concentration. The above-mentioned nickel-manganese mixed solution, compounding agent solution, and precipitant are continuously added to the reactor respectively, the total amount of nickel-manganese mixed solution added is 10 liters, and the total amount of compounded agent solution added is based on the total molar amount of solute in the compounded agent solution Calculated as 0.2 times the total molar am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
| First discharge capacity | aaaaa | aaaaa |
| First discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com