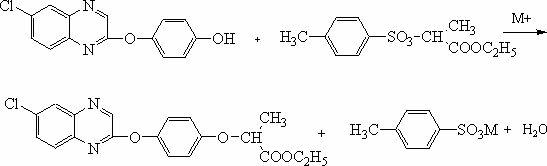
High yield synthetic method for quizalofop-p-ethyl
A synthetic method, the technology of quizalofop-p-ethyl, which is applied in the field of pesticide product synthesis, can solve the problems of large solvent loss, high production cost, and long production cycle, and achieve the effects of less side reactions, short process, and reduced equipment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Put 275kg of 6-chloro-2-(4-hydroxyphenoxy)quinoxaline with a content of 97.4% in a 5000L reactor with a water separation device, and pay attention to prevent static electricity with anti-static clips, and then put in sodium carbonate 250kg, open the solvent pipeline valve and inject 2750kg cyclohexane to close the manhole. In the metering tank, 298 kg of R (-) ethyl p-toluenesulfonyl lactate with a vacuum extraction content of 96% was used for subsequent use.
[0026] After the crank is flexible, start stirring, heat up and reflux to carry water until no more water droplets sink in the water separator, continue to carry water for 0.5 hours, and the reaction system is in an anhydrous state.
[0027] After the addition of water, keep the system in the state of reflux with water, and add R(-) ethyl p-toluenesulfonyl lactate dropwise at a uniform speed, which takes 4.5 hours. After the addition is completed, continue to react for 6 hours, and take samples for tracking.
[...
Embodiment 2
[0034] Put 400kg of 6-chloro-2-(4-hydroxyphenoxy)quinoxaline with a content of 97% in the 5000L reactor, pay attention to prevent static electricity with anti-static clips, then put in 230kg of potassium hydroxide, and open the solvent pipeline The valve measures 2400kg cyclohexanone as the reaction solvent, and the manhole is closed. In the metering tank, 423 kg of R (-) ethyl p-toluenesulfonyl lactate with a vacuum extraction content of 96.3% was used for subsequent use.
[0035] After the crank is flexible, start stirring, heat up and return to bring water until the water separator no longer sinks, continue to carry water for 0.5 hours, and the reaction system is in an anhydrous state. After carrying water, keep the system in the state of reflux with water, and add R(-) ethyl p-toluenesulfonyl lactate dropwise at a uniform speed, which takes 2 hours, and take samples for tracking after 10 hours of reaction. With gas chromatography analysis, 6-chloro-2-(4-hydroxyphenoxy gro...
Embodiment 3
[0040] Put 275kg of 6-chloro-2-(4-hydroxyphenoxy)quinoxaline with a content of 96.5% into the 5000L reactor, pay attention to prevent static electricity with anti-static clips, then put in 163kg of potassium hydroxide, and open the solvent pipeline Inject 2500kg cycloheptane into the valve to close the manhole. In the metering tank, 310 kg of R (-) ethyl p-toluenesulfonyl lactate with a vacuum extraction content of 95.8% was used for subsequent use.
[0041]After the crank is flexible, start stirring, heat up and return to bring water until the water separator no longer sinks, continue to carry water for 0.5 hours, and the reaction system is in an anhydrous state. After the addition of water, keep the system in the state of reflux with water and add R(-) ethyl p-toluenesulfonyl lactate dropwise at a constant speed for 4 hours. After the dropwise addition, continue to react for 4 hours to take samples for tracking. Analyze the content of 6-chloro-2-(4-hydroxyphenoxy)quinoxalin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com