Human prolactin receptor antibody and application thereof
A prolactin receptor and antibody technology, applied in the field of genetic engineering technology and biological products, can solve the problem of breast cancer without effective treatment methods, and achieve the effect of highly targeted application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Separation of human peripheral blood lymphocytes and extraction of total RNA
[0039] Collect 2 to 40 samples of anticoagulated peripheral blood in batches from patients aged 20 to 60 years old and clinically diagnosed with breast cancer. Collect peripheral blood lymphocytes with lymphocyte separation medium to obtain peripheral blood lymphocytes, mix them, and wash with sterilized PBS. The cells were resuspended, and the total RNA of peripheral blood lymphocytes was extracted with the total RNA purification kit.
Embodiment 2
[0040] Example 2: Amplification of human immunoglobulin G (IgG) light chain and heavy chain Fd segment genes
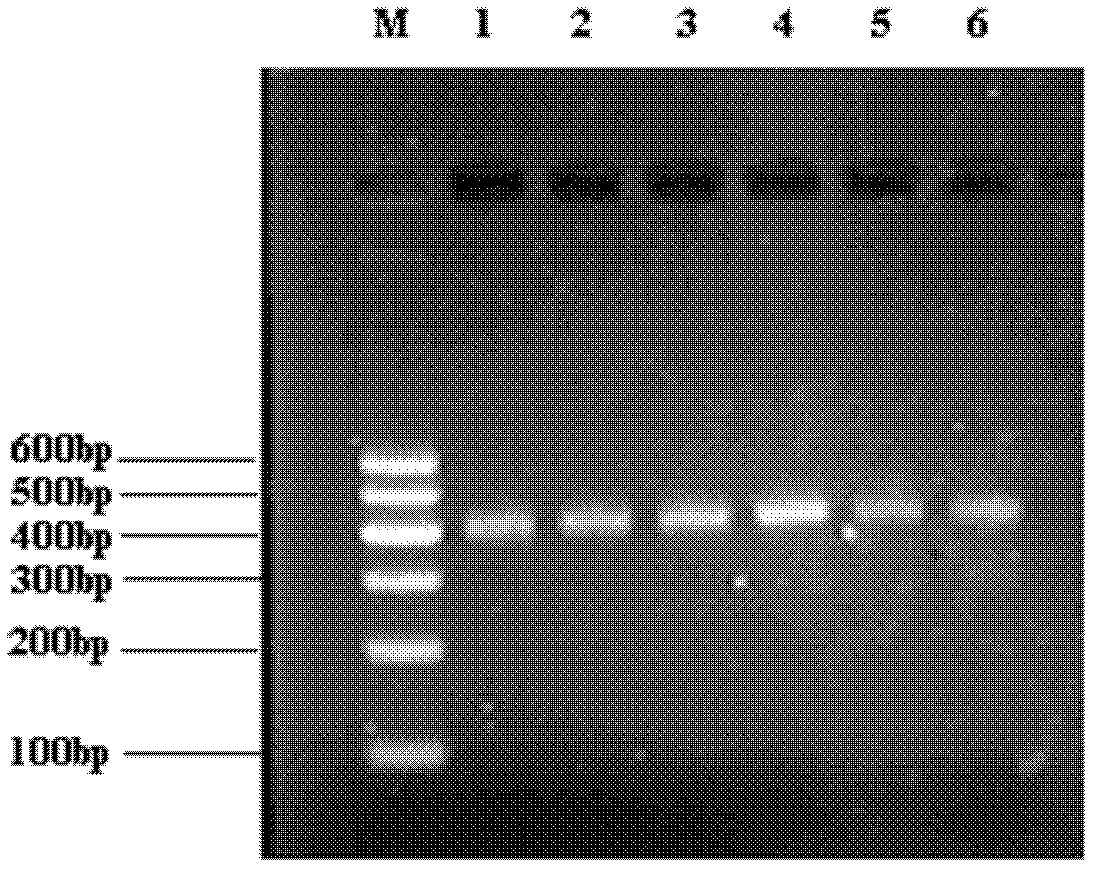
[0041] First, the total RNA of lymphocytes is reverse-transcribed into cDNA, and then a group that almost covers the human immunoglobulin G (IgG) light chain or heavy chain Fd segment V H and C H 1 Specific primers for the coding sequences of the variable region and the constant region of the gene fragment, and PCR was performed on the above reaction products to amplify human immunoglobulin G (IgG) respectively, using 4 pairs of κ chain gene primers and 9 pairs of λ chain gene primers respectively And 6 pairs of heavy chain gene primers to amplify the variable region. The amplification conditions were 94°C for 5 minutes, 94°C for 1-2 minutes, 50-60°C for 1-2 minutes, and 72°C for 1-2 minutes, a total of 30 cycles, and the final extension was 72°C for 10 minutes. PCR products were identified by 2.0% agarose gel electrophoresis. The amplification results of 6 pairs o...
Embodiment 3
[0061] Example 3: Construction of natural human anti-breast cancer IgG Fab phage antibody library
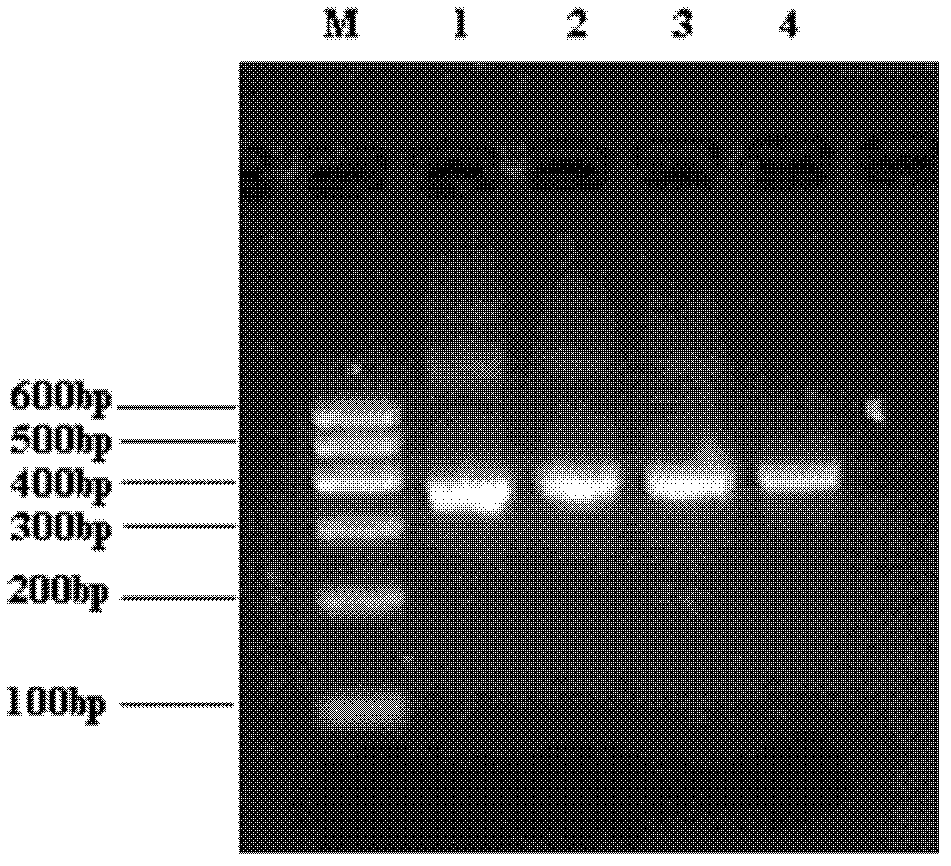
[0062] Using the strategy of first cloning the light chain gene and then the heavy chain gene, Fab fragments targeting breast cancer patients with good diversity were obtained by overlapping PCR ( Figure 4 ), the Fab length is about 1600bp, the Fd segment of the heavy chain and the light chain gene are about 750bp, and the variable and constant regions of the light chain and the heavy chain are about 350bp.
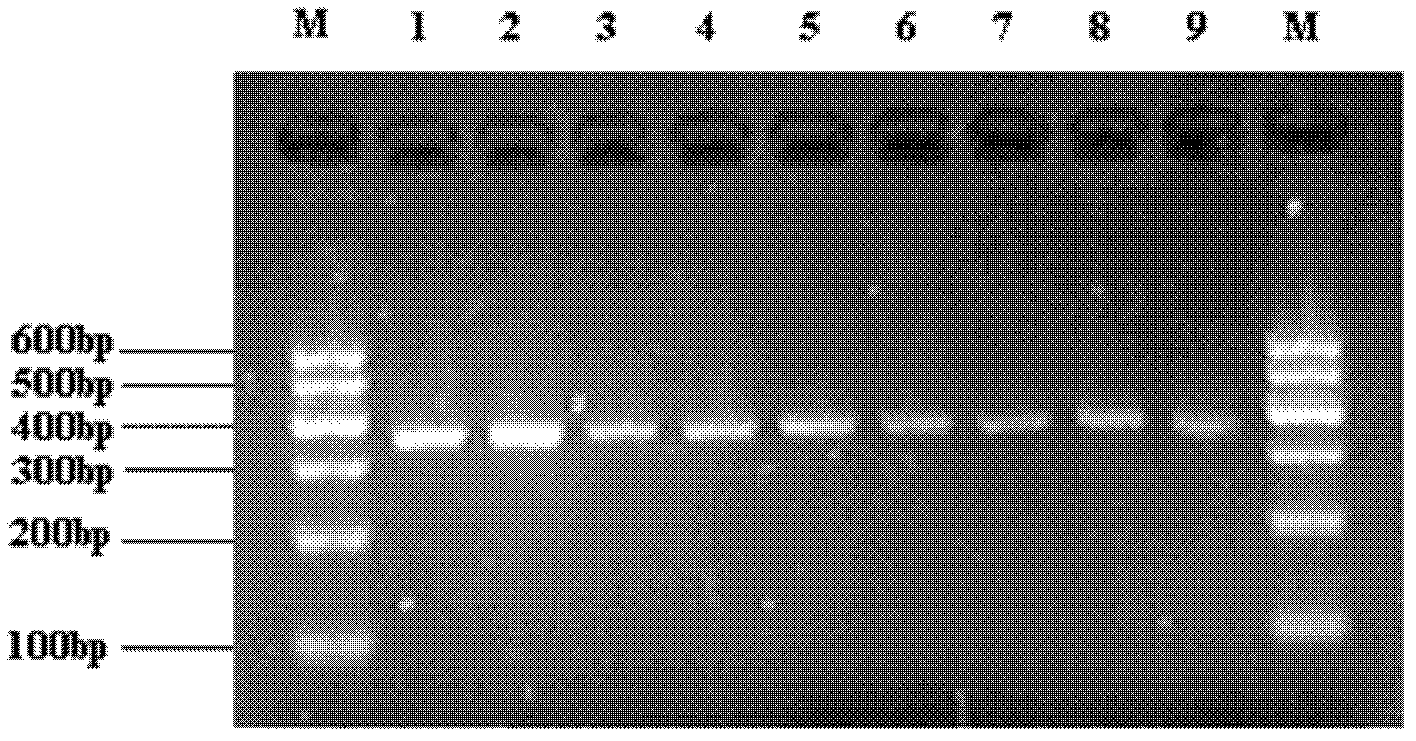
[0063] Then the Fab fragment and plasmid pcomb3XSS were digested with Sfi I, and the fragment of the PCR product was digested with the phage vector pCom3XSS (gifted by Scripps Institute, Figure 5 ) connection, after electroporation into competent cells, spread the transformed bacteria evenly on LB plates containing 100 μg / ml ampicillin, collect the plasmid DNA of all clones, and construct a library with a capacity of 1×10 9 Human breast cancer IgG Fab phage antibody libr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com