Pharmaceutical preparation comprising supernatant of blood mononuclear cell culture
A supernatant, nuclear cell technology, used in drug combinations, surgical drugs, antipyretics, etc., can solve problems such as tissue damage that does not directly solve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
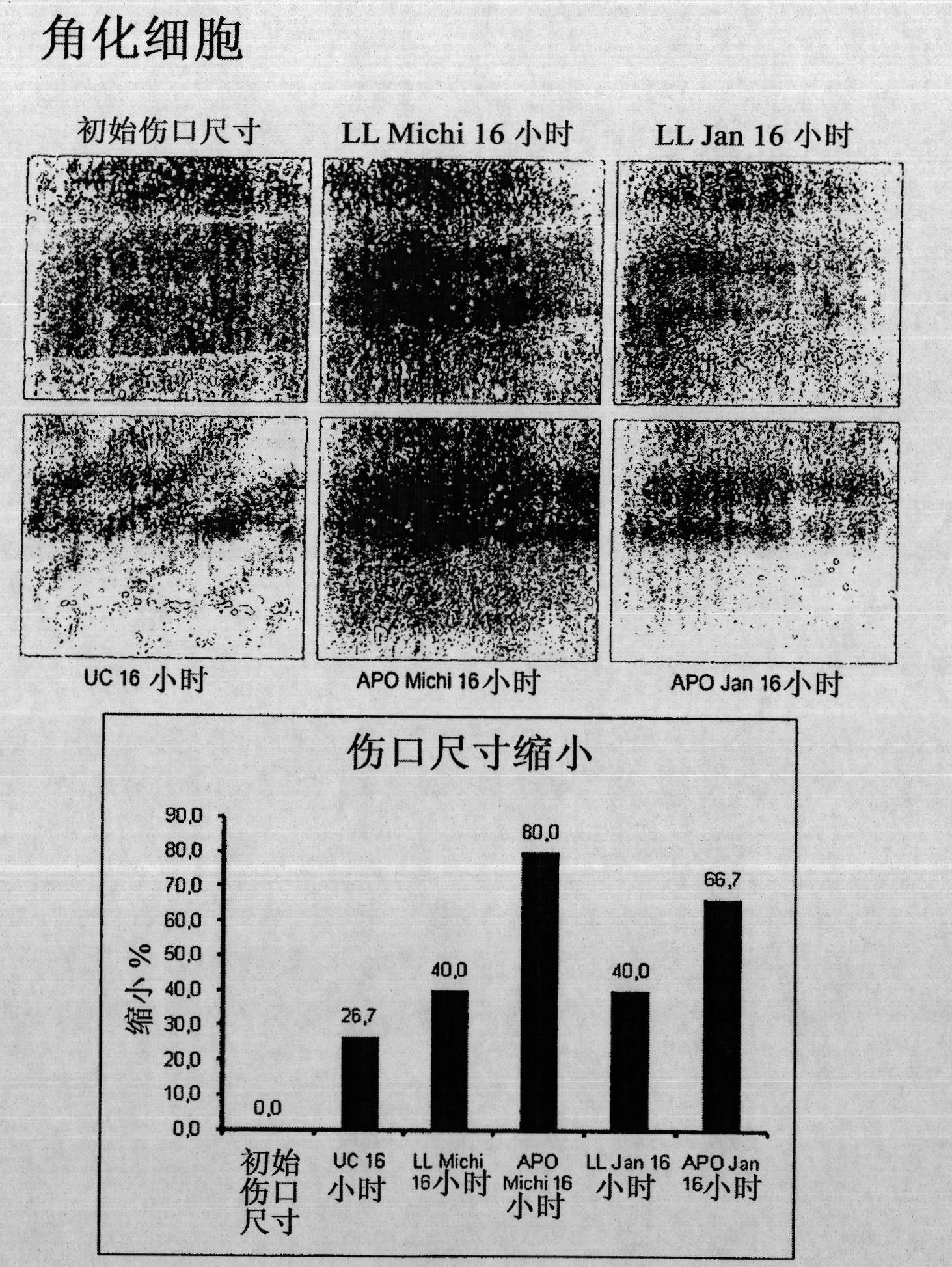
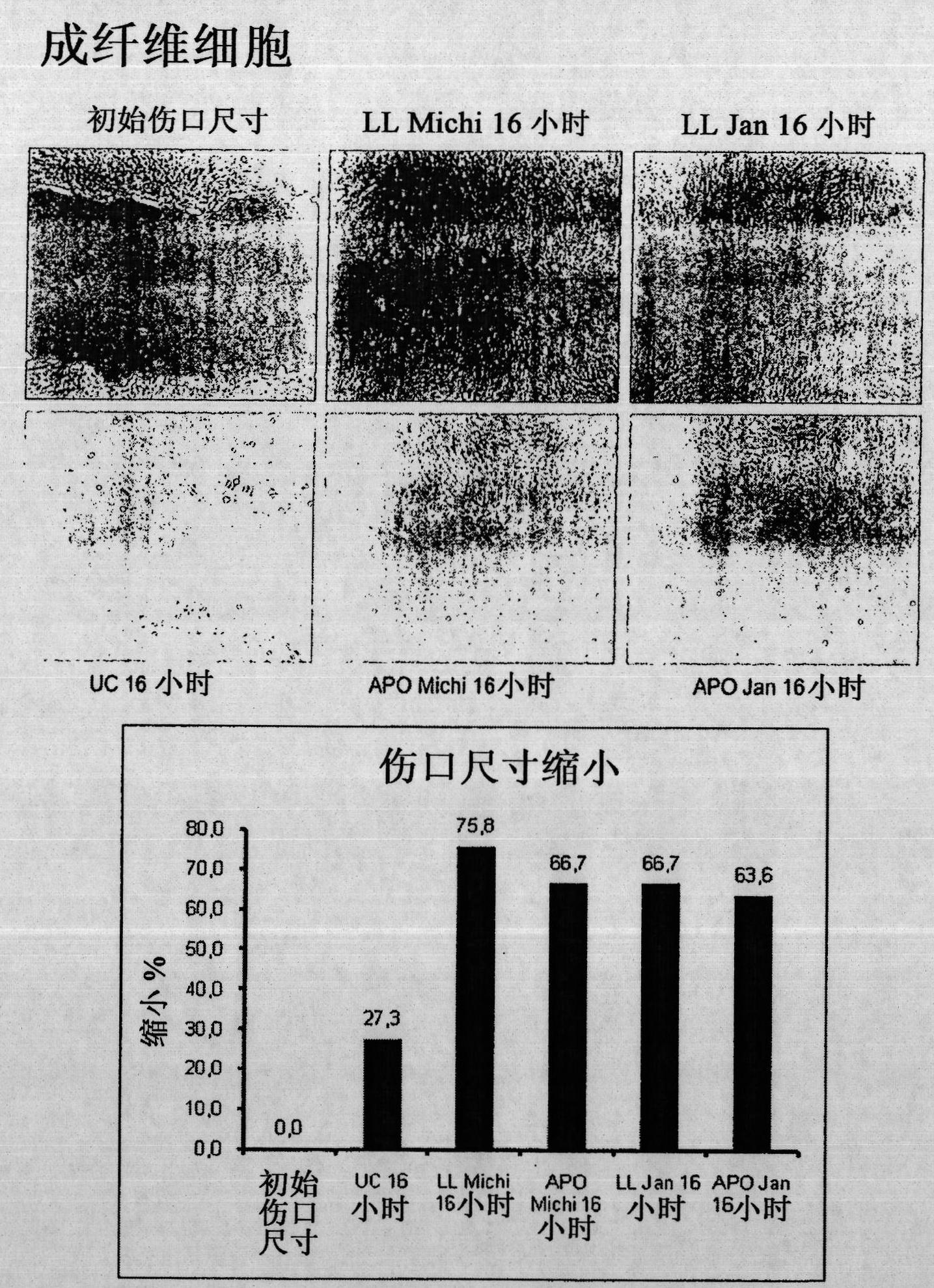
[0075] Example 1: Culture supernatants of peripheral blood mononuclear cells strongly enhance wound healing.
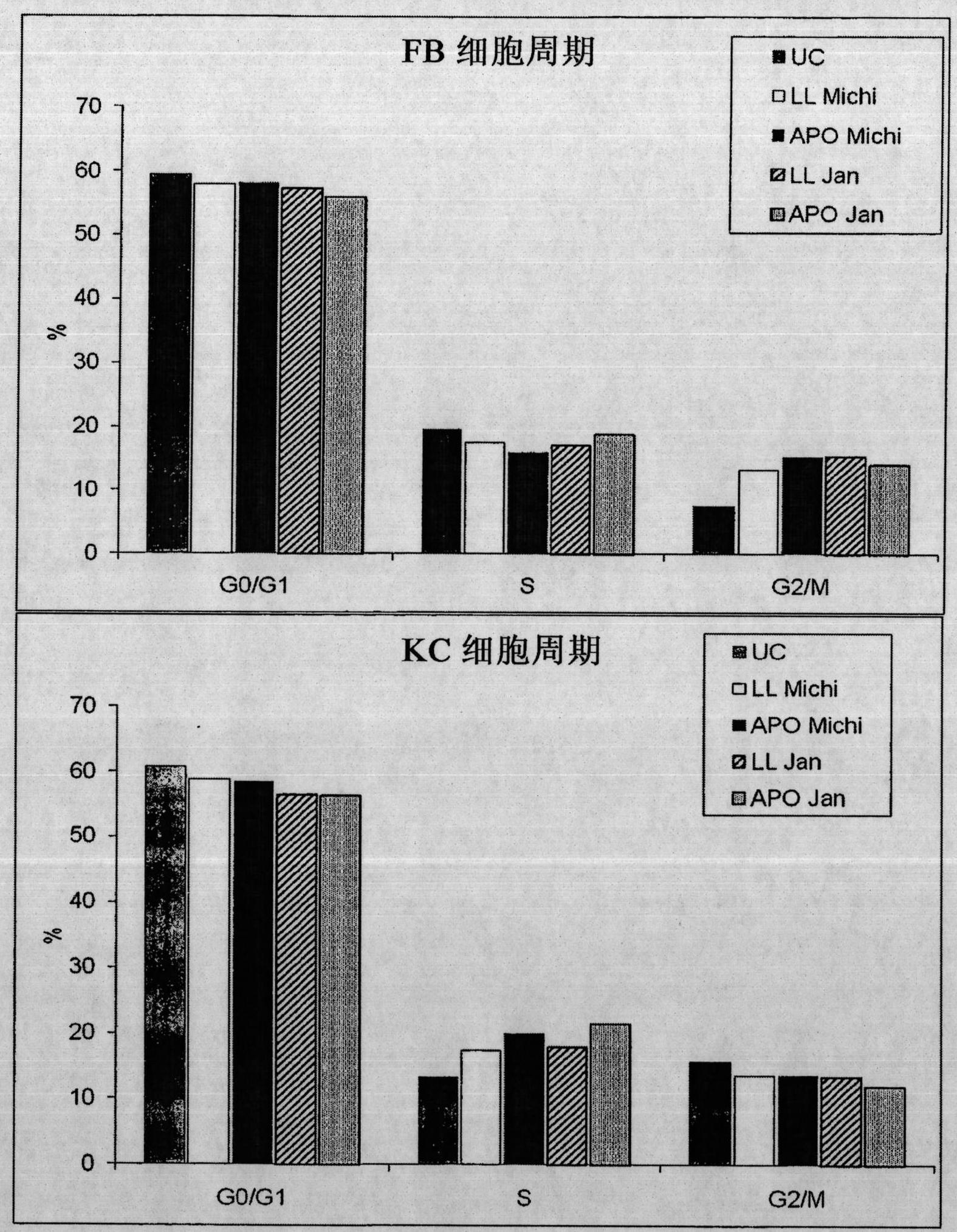
[0076]Unhealed skin ulcers are often resistant to most common treatments. Previous studies have shown that the administration of peripheral blood mononuclear cells (PBMCs) together with basic fibroblast growth factor is a useful treatment for diabetic gangrene. In this example it was investigated whether culture supernatants of PBMCs (non-irradiated or irradiated) were sufficient to induce enhanced wound healing in a mouse model. Furthermore, the effect of these supernatants on human primary fibroblasts (FB), keratinocytes (KC) and endothelial cells (EC) was analyzed.
[0077] Incubation of FB and KC with PBMC-derived supernatants revealed that both non-irradiated and irradiated cell supernatants strongly induced FB migration, but they had no effect on FB proliferation. In contrast, both supernatants were shown to be effective on the migratory and proliferative ca...
Embodiment 2
[0079] Example 2: Quiescent Peripheral Blood Mononuclear Cells (PBMCs) Demonstrate Low Activation Markers and Reduced Inflammatory Cells cytokine production
[0080] Activated peripheral blood mononuclear cells (PBMC) and their supernatant (SN) are thought to be beneficial in wound regeneration (Holzinger C et al. Eur J Vasc Surg. 1994 May;8(3):351-6). In Examples 1 and 2, it was shown that unactivated PBMCs and their derived SNs have beneficial effects in experimental acute myocardial infarction (AMI) and trauma models. Since non-activated PBMCs had to be confirmed experimentally, it was investigated whether culture of PBMCs resulted in enhanced T cell activation markers (CD69, CD25) or enhanced secretion of inflammatory cytokines (monocyte activation = TNFα, T cell activation =INFγ). In control experiments, cultured T cells were stimulated by CD3 mAb stimulation or phytohemagglutinin (PHA).
[0081] Method and Results
[0082] Venous blood was collected from healthy...
Embodiment 3
[0091] Example 3: Proliferative Activity of PBMCs Cultured in Physiological Solution
[0092] The purpose of this example was to demonstrate that specific (CD3), non-specific (lectin, PHA) and allogeneic T cells were utilized in comparison to 2-day (CD3, PHA) and 5-day (MLR) stimulation assays Triggered (Mixed Lymphocyte Reaction, MLR) immunoassay, PBMC had no proliferative activity.
[0093] Materials and methods
[0094] PBMCs were isolated from young healthy volunteers by Ficoll density gradient centrifugation and 1*10 per 200 μL 5 Each cell was resuspended in RPMI (Gibco, USA) containing 0.2% gentamicin sulfate (Sigma Chemical Co, USA), 1% L-glutamine (Sigma, USA). Responder cells were treated with anti-CD3 MoAb (10 μg / mL, BD, NJ, USA), PHA (7 μL / mL, Sigma Chemical Co, USA), or with irradiated allogeneic PBMC at a 1:1 ratio (for MLR )Stimulate. Incubate the plate for 48h or 5 days, then use 3 [H]-thymidine pulse 18h (3.7*10 4 Bq / well; Amersham Pharmacia Biotech, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com