A kind of synthetic method of trihydrate 3-aminopropylamine ethylthiophosphoric acid
A technology of aminopropylamine ethylthiophosphoric acid and its synthesis method, which is applied in the field of synthesis of trihydrate 3-aminopropylamineethylthiophosphoric acid to achieve good biocompatibility, high purity and high purity of the crude product Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
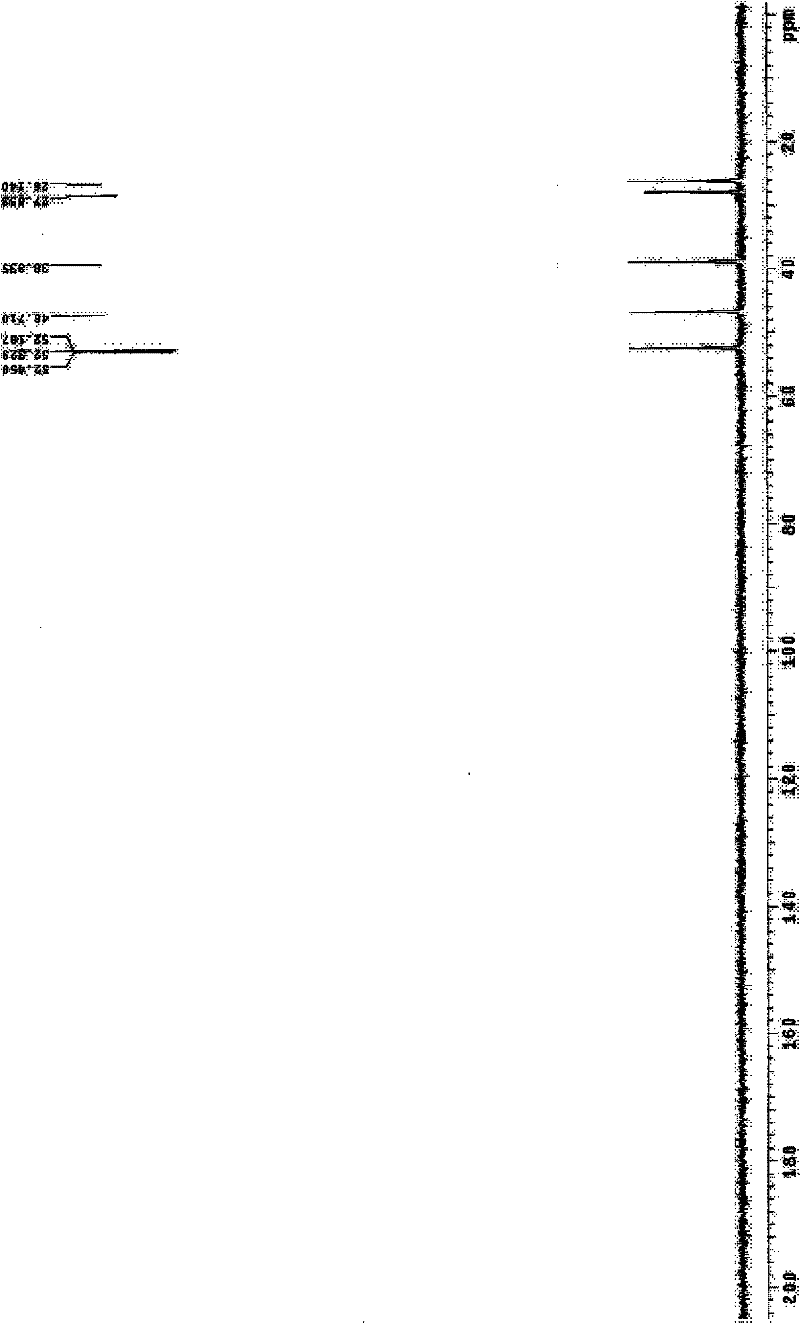
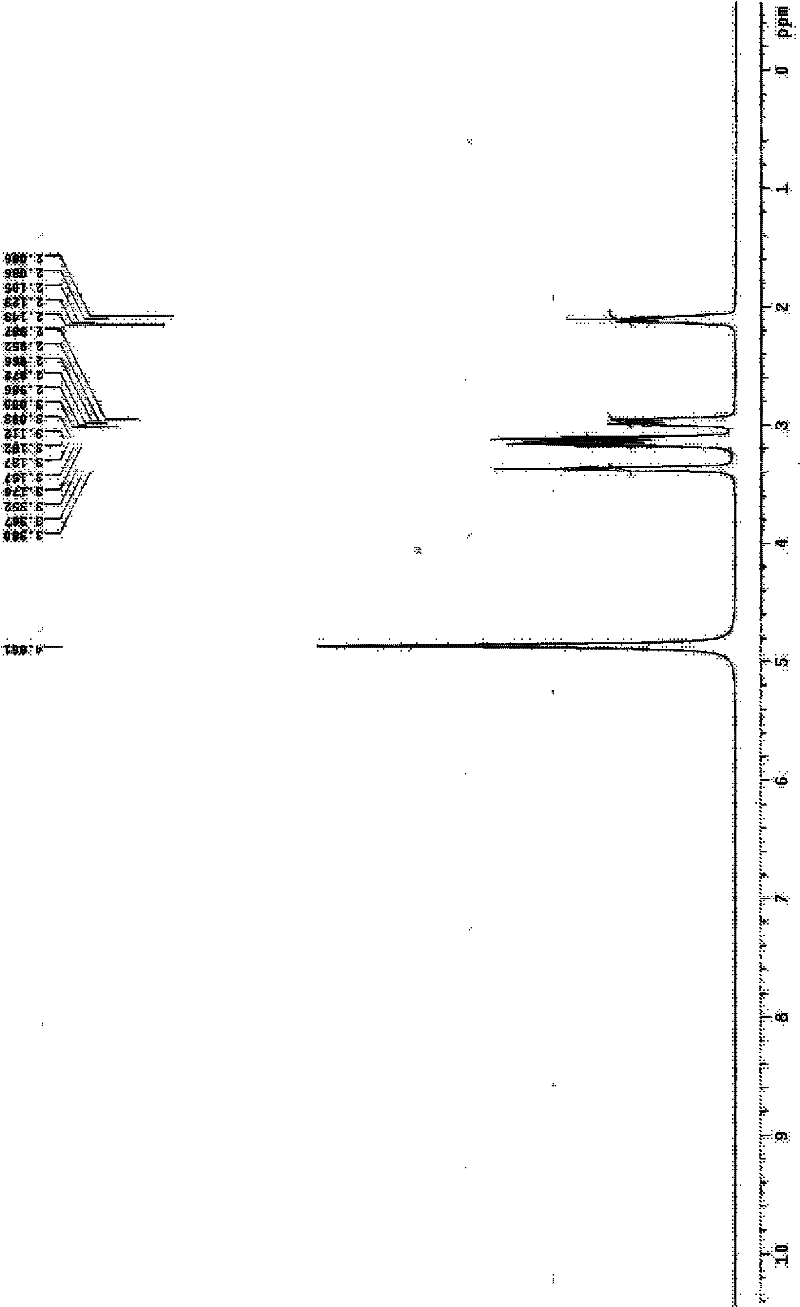
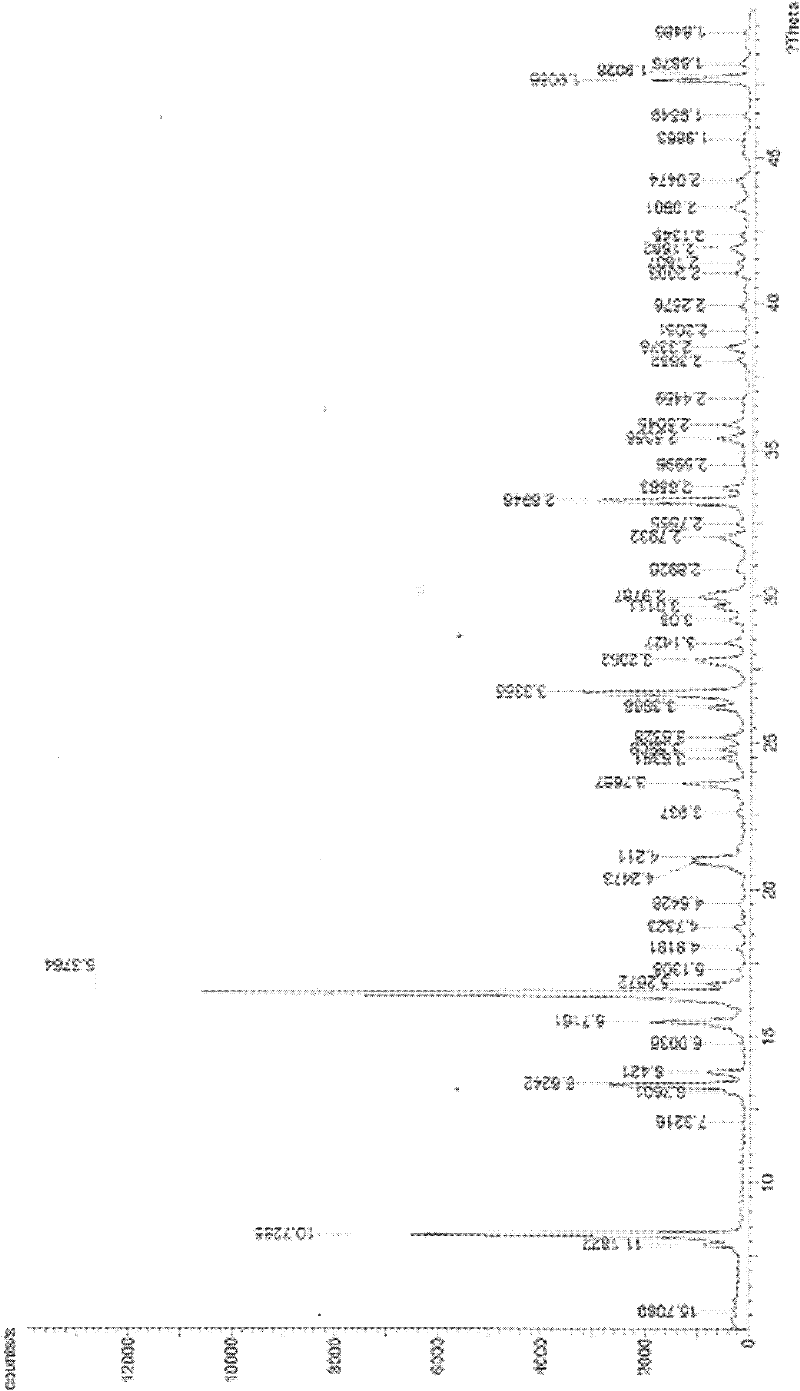
Image
Examples
Embodiment 1
[0040] Embodiment 1 Amifostine synthesis process of the present invention
[0041] a. Add sodium thiophosphate dodecahydrate (10.0 g, 25.2 mmol) and 25 ml of distilled water into a flask equipped with an internal temperature thermometer, stir and suspend, and place in an external ice-water bath. First add N-(2-bromoethyl)-1,3-propanediamine dihydrobromide (9.5g, 27.7mmol) to obtain a light brown suspension system, then add polyethylene glycol 400, namely PEG400 ( 10 g), stirred evenly, removed the ice-water bath, and kept stirring and reacting at 5-10°C. During the reaction, the reaction system first turned into a light brown clear solution, and then turned into a near-white turbid system again.
[0042] b. After reacting for 24 hours, add 163ml of concentrated ammonia water-methanol mixture (volume ratio 1 / 29) to the stirred reaction material at room temperature, and a white solid can be seen in the bottle. After stirring for about 10 min, stand at 4°C for crystallization. ...
Embodiment 2
[0043] Embodiment 2 Amifostine synthesis process of the present invention
[0044] First synthesize according to the technique of embodiment 1 and obtain the amifostine crude product, then carry out the following operations to refine:
[0045] One-time refining: Take 1 g of the above crude product, add 10 ml of aqueous solution containing 5% ethanol to dissolve, add 0.02 g of activated carbon and 1.0 g of 201×7 type strongly acidic anion exchange resin, stir in an oil bath at 30°C for 10 min, filter under normal pressure, and add water The phase was kept and stirred in a 30°C oil bath, 4ml of 99.5% ethanol was added dropwise, and then the bath temperature was gradually decreased from 30°C to room temperature. At this time, a large amount of white solids had precipitated, and then stood at 4°C for 2h, filtered with suction, and vacuum-dried at 30°C to obtain 0.78g of a white primary refined product, with a yield of 78%, and a content of about 98% as determined by HPLC.
[0046...
Embodiment 3
[0047] Example 3 Screening test under different conditions of amifostine synthesis process of the present invention
[0048] The following tables are the experimental results obtained when preparing the crude product of amifostine according to the operation process of step a in Example 1 using different proportioning ratios and reaction temperatures (the method of step b is the same)
[0049] Table 1
[0050]
[0051] As can be seen from Table 1, the consumption ratio of water and PEG plays an important role in the yield and purity of the reaction: when the weight ratio of sodium salt and bromine salt was at 1: 0.8-1.3, the weight ratio of water and PEG was at 4 :1~1:3, the yield of product is all above 54%, and the purity is higher than 73%; When the weight ratio of water and PEG is at 4:1~1.6:1, the yield of product is all above 83% , and the purity is higher than 87%, showing that this condition is better than the existing pure water reaction system (such as Li Jiaming,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com