Bimetallic heteroligand catalyst precursor and its synthesis method and application
A technology of catalysts and heteroligands, applied in the application of catalyzed olefin polymerization and copolymerization, the synthesis of bimetallic heteroligand catalyst precursors, and the field of catalysts, can solve problems such as the inability to effectively regulate the proportion of comonomers, and achieve The effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
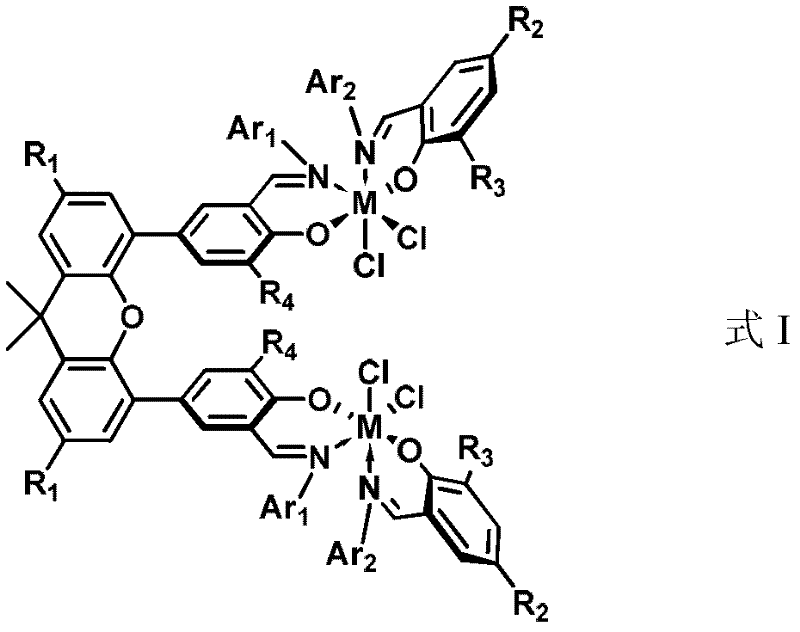
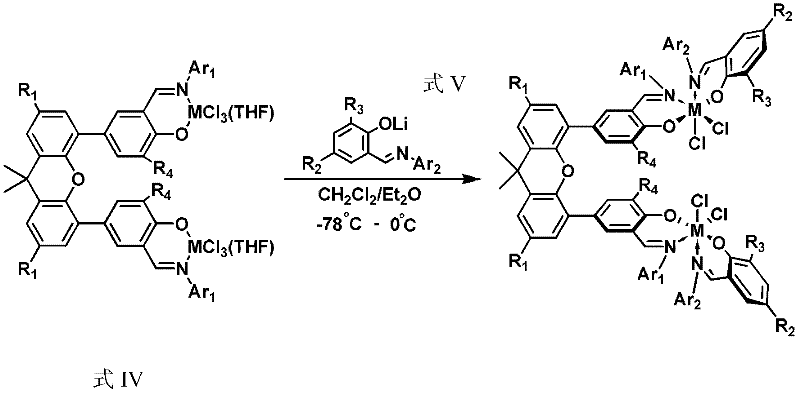
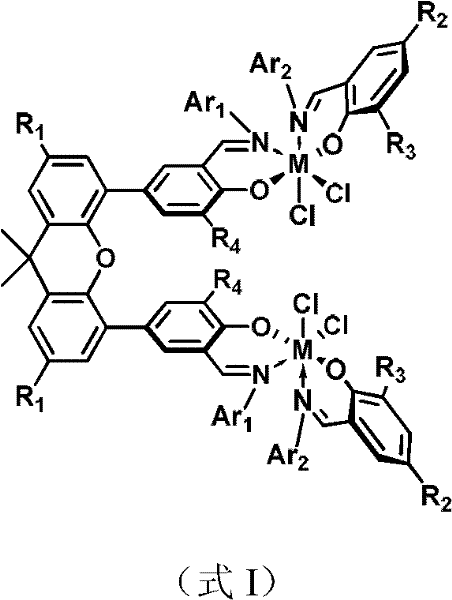
[0032] 1) the preparation of the bimetallic heteroligand catalyst precursor shown in formula II
[0033]Dissolve (E)-2-tert-butyl-6-((pentafluorophenylimino)methyl)phenol (0.77g, 2.24mmol) in ether solvent, and add n-BuLi (1.67M n-hexane solution, 2.35 mmol) was reacted for 1 hour, returned to room temperature, and continued to react for 15 minutes. Afterwards, the solution was transferred to a dichloromethane solution of a titanium metal complex (1.50 g, 1.12 mmol) with tetrahydrofuran at -78 ° C through a double-angle needle, and reacted at this temperature for 4 hours, and then the system was gradually Return to room temperature and react for another 12 hours. After the reaction, the solvent was removed with a vacuum line, the residue was washed with dichloromethane and filtered through celite, the filtrate was sucked dry, and the crude product was recrystallized with dichloromethane / ethyl ether / n-hexane to obtain reddish-brown needle crystals (0.45 g, 22%). 1 H NMR (300...
Embodiment 2
[0037] Synthesis of polyethylene: evacuate the heated and dried 250mL polymerization bottle with nitrogen for two times, then evacuate with ethylene gas, then add 5mL of methylalumoxane (MAO) toluene solution (12mg / mL) in sequence , 43 mL of anhydrous and oxygen-free toluene, 2 mL of toluene solution of metal catalyst II (0.9 mg / mL). Feed ethylene with a pressure of 1 atm under magnetic stirring, and react at 20°C for 5 min under this pressure, then add an acidified solution of ethanol to terminate the reaction, and obtain 0.615 g of a polymer with an activity of 3.7×10 6 g·mol -1 (Ti)·h -1 .
[0038] The melting point measured by DSC is 139.1°C; the M value of polyethylene measured by GPC w 1.9×10 5 ,M w / M n is 1.82.
Embodiment 3
[0040] Synthesis of polyethylene: evacuate the 250mL polymerization bottle after heating and drying, and pass nitrogen gas twice, and then pass ethylene gas after vacuuming, and then add 2.5mL (12mg / mL) toluene solution of methylaluminoxane (MAO) in sequence ), 45.5 mL of toluene treated with anhydrous and oxygen-free treatment, and 2 mL of toluene solution of metal catalyst II (0.9 mg / mL). Under magnetic stirring, ethylene with a pressure of 1 atm was introduced, and reacted at 20°C for 5 minutes under this pressure, and then an acidified solution of ethanol was added to terminate the reaction to obtain 0.632 g of a polymer with an activity of 3.8×10 6 g·mol -1 (Ti)·h -1 .
[0041] The melting point measured by DSC is 138.9°C; the M of polyethylene measured by GPC w 1.8×10 5 ,M w / M n is 1.77.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com