A kind of solid-phase synthesis method of ziconotide
A technology of solid-phase synthesis and ziconotide, which is applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of low yield, low accuracy of disulfide bond connection, and great difficulty in separation and purification, etc. problems, to achieve the effect of streamlining steps, easy preparation and purification, and the same number of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
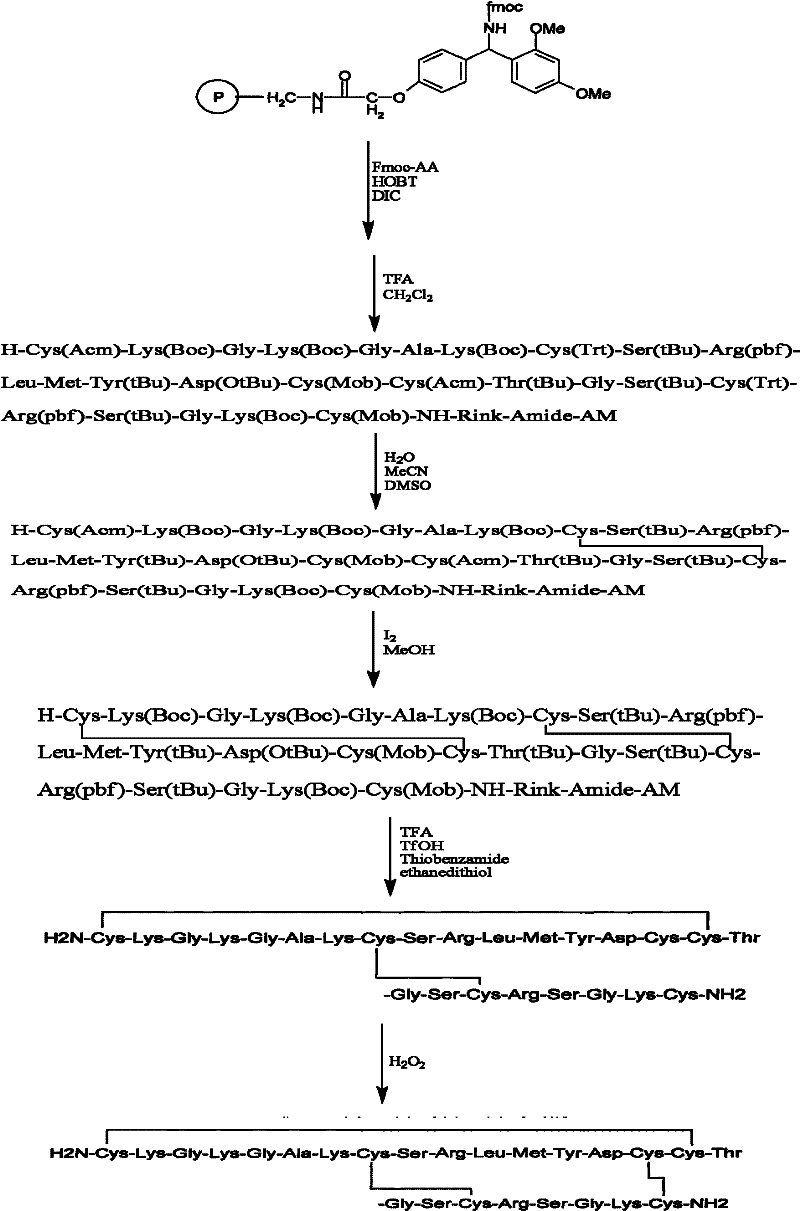
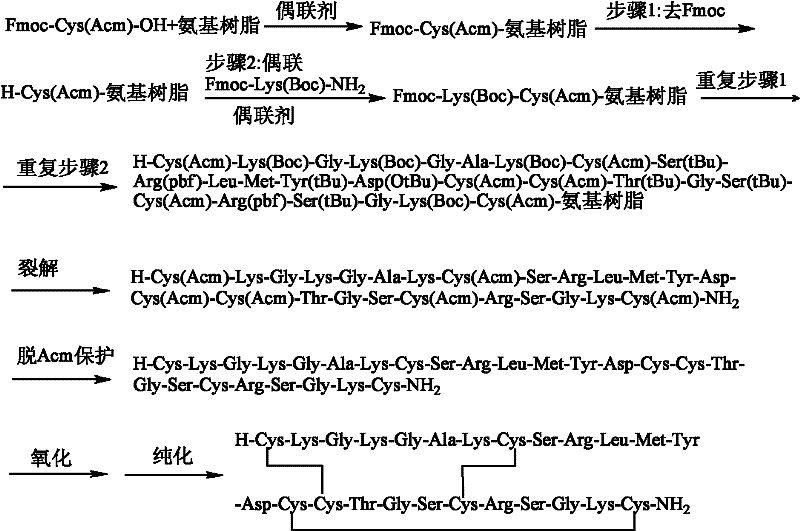
[0040] Example 1: Preparation of Ziconotide Linear Peptide Resin.
[0041] Resin swelling: Weigh 1g of Fmoc-Rink-Amide-MBHA resin and place it in a 20ml BD syringe with a sieve plate (ordinary glass peptide reactor is also acceptable), and use 3 times the volume of the resin to swell with DCM for 1-4 hours until the resin is completely swollen After that, 20% (v / v) piperidine / DMF solution was used to remove the Fmoc protecting group twice for 5 min and 20 min respectively, and washed 6 times alternately with DCM and DMF.
[0042] Amino acid connection: Dissolve 2mmol of Fmoc-protected amino acid, 2mmol of HoBt, 2mmol of HBTU, and 4mmol of DIPEA in DMF, preactivate for 5 minutes, then add to the swollen resin (resin mass*substitution value=0.5mmol), shake at room temperature React for 1.5-2.5 hours, see Table 1 for the dosage and specific reaction time of each amino acid and condensing agent. Remove the Fmoc protection group before connecting the next amino acid with piperidin...
Embodiment 2
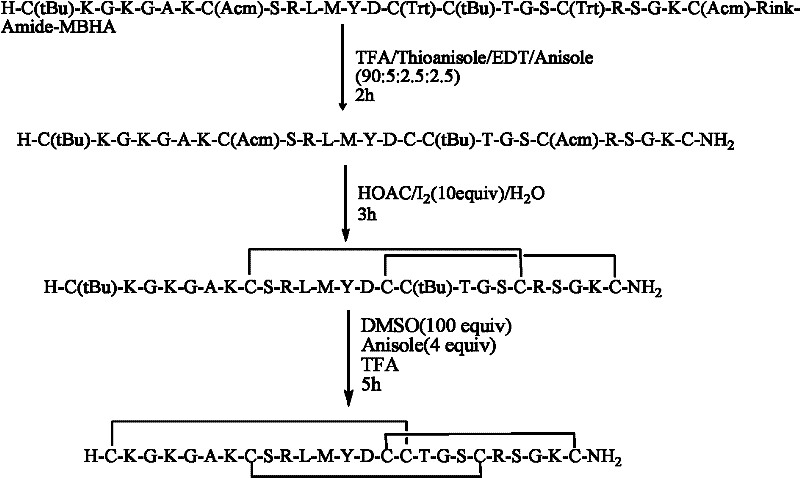
[0049] Example 2: Cleavage of resin and removal of some side chain protecting groups.
[0050] Mix trifluoroacetic acid, thioanisole, dimercaptoethane and anisole in a volume ratio of 90:5:2.5:2.5, or mix trifluoroacetic acid, methanol, water and triisopropylsilane in a volume ratio Mix 88:5:5:2, add the linear fully protected peptide resin prepared in Example 1 to any one of the above two mixed solutions, use a volume of 10 mL of the mixed solution per 1 g of linear fully protected peptide resin, shake After 2 hours of reaction, the reaction solution was poured into -20°C cold diethyl ether for precipitation. After centrifugation, the white precipitate was collected and air-dried to obtain a linear crude peptide containing Acm and tBu in the Cys side chain, weighing 1.4 g, and the crude yield was 94%.
Embodiment 3
[0051] Example 3: Formation of Ziconotide Bicyclic Peptide.
[0052] 1.0 g of the linear peptide prepared in Example 2 was dissolved in acetic acid so that the concentration of the linear peptide was 0.5 mg / mL, and then 10 times the molar amount of I 2 (dissolved in methanol), stirred and reacted at room temperature for 1 hour, then added 20% water of the reaction solution volume, and whether the reaction was complete was detected by HPLC. After the reaction was complete, 0.1M ascorbic acid was added until the solution became colorless, and the reaction mixture was frozen. After drying, the precipitate was washed with ether to obtain a crude bicyclic peptide containing tBu in the Cys side chain, weighing 0.91 g, and the crude yield of this step was 91%.
[0053] Analytical HPLC detection method is:
[0054] Equipment: C18 analytical column: 4.6×150mm;
[0055] Eluent A: 0.1% (v / v) TFA / H 2 O;
[0056] Eluent B: 0.08% (v / v) TFA / acetonitrile;
[0057] Flow rate: 1ml / min;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com