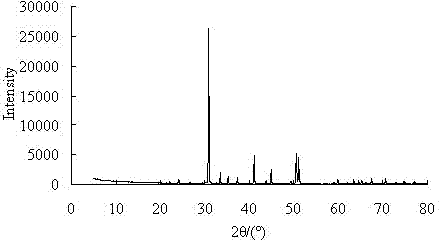
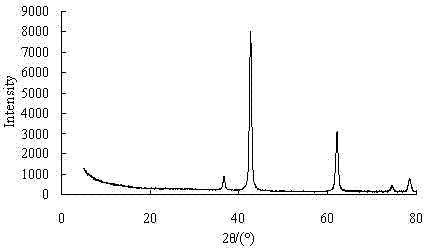
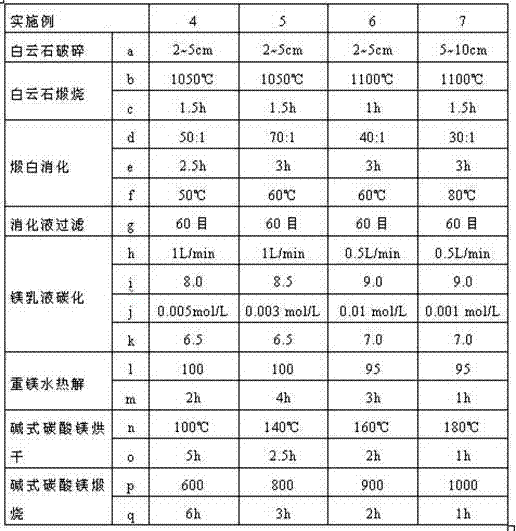
Process for producing high purity magnesium oxide with dolomite
A process method, the technology of dolomite, applied in the direction of magnesium oxide, etc., can solve the problem of difficult separation of calcium and magnesium, and achieve the effect of simple process, high purity, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: The chemical composition of dolomite is MgO content 20.96%, CaO content 30.06%, broken to 2~5cm. Calcined at 900°C for 3h to obtain calcined white. The calcined white is mixed according to the mass ratio of water: calcined white is 50:1, heated and stirred to 60° C., kept for 1 hour for digestion, and the emulsion is obtained. The emulsion is passed through a 60-mesh sieve to obtain refined magnesium emulsion. After cooling to room temperature, CO 2 , CO 2 The flow rate is 0.5L / min, and the pH value of the carbonization system is tracked and tested. When the carbonization system is at pH=8.5, add ammonium oxalate to make the concentration of oxalate in the system 0.002mol / L, continue carbonization to pH=6.5, and stop carbonization. The carbonized system is separated from solid to liquid, the solid phase is light calcium carbonate, and the liquid phase is heavy magnesium water. The obtained heavy magnesium water is pyrolyzed at 95°C for 2 hours, solid-liq...
Embodiment 2
[0033] Example 2: The composition of dolomite is MgO content 20.96%, CaO content 30.06%, broken to 2~5cm. Calcined at 950°C for 2.5h to obtain calcined white. Mix calcined white according to the mass ratio of water: calcined white is 40:1, stir and heat to 70°C, heat-preserve and digest for 1.5h to obtain emulsion. The emulsion is passed through a 60-mesh sieve to obtain refined magnesium emulsion. After cooling to room temperature, CO 2 , CO 2The flow rate is 0.8L / min, and the pH value of the carbonization system is tracked and tested. When the carbonization system is at pH=9.0, add oxalic acid to make the concentration of oxalate in the system 0.005mol / L, continue carbonization to pH=6.8, and stop carbonization. The carbonized system is separated from solid to liquid, the solid phase is light calcium carbonate, and the liquid phase is heavy magnesium water. The obtained heavy magnesium water is pyrolyzed at 100°C for 2 hours, solid-liquid separation after cooling, and t...
Embodiment 3
[0034] Example 3: The composition of dolomite is MgO content 20.96%, CaO content 30.06%, broken to 2~5cm. Calcined at 1000°C for 2 hours to obtain calcined white. Mix calcined white according to water: calcined white = 60:1, stir and heat to 70°C, heat-preserve and digest for 2 hours to obtain emulsion. The emulsion is passed through a 60-mesh sieve to obtain refined magnesium emulsion. After cooling to room temperature, CO 2 , CO 2 The flow rate is 1L / min, and the pH value of the carbonization system is tracked and tested. When the carbonization system is at pH=9.0, add oxalic acid to make the concentration of oxalate in the system 0.008mol / L, continue carbonization to pH=6.5, and stop carbonization. The carbonized system is separated from solid to liquid, the solid phase is light calcium carbonate, and the liquid phase is heavy magnesium water. The obtained heavy magnesium water is pyrolyzed at 100°C for 2 hours, solid-liquid separation after cooling, and the solid phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com