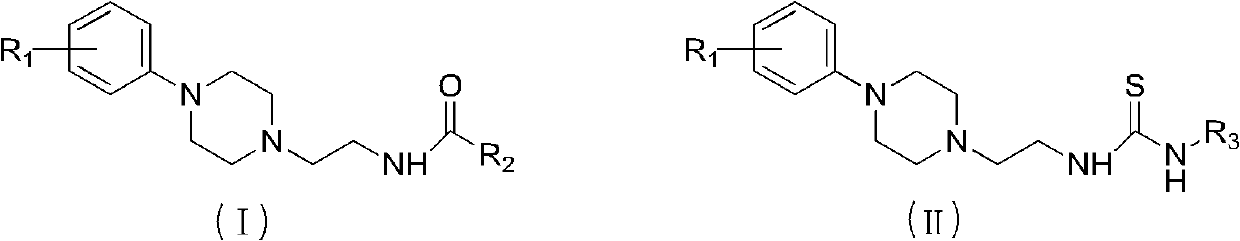
A small molecule fluorescent probe of phenylpiperazine α1-adrenoceptor and its application
A kind of epinephrine and fluorescent probe technology, applied in the field of medicine, can solve the problems of the research and development of biological and pharmacological properties lagging behind
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
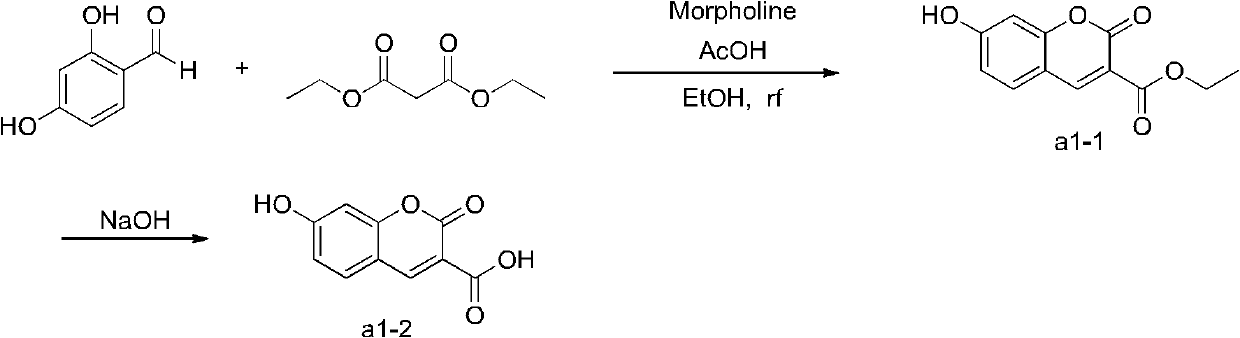
[0020] Example 1: N-{2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl}-7-hydroxyl-1,2-benzopyrone-3-formamide ( Preparation of b1)
[0021] Preparation of intermediate a1-2:
[0022]
[0023] Preparation of intermediate (a1-1):
[0024] 100mL flask, add 2.76g 2,4-dihydroxybenzaldehyde and 25mL absolute ethanol, stir to make the solution clear, then add 3.84g diethyl malonate, while stirring, dissolve 0.17g morpholine and 66mg acetic acid In 10 mL of absolute ethanol, it was added to the above-mentioned reaction solution, refluxed and stirred for 9 h, the reaction solution was cooled in an ice bath, a large amount of precipitates precipitated, filtered, and dried to obtain 2.37 g of yellow needle-like solids with a yield of 50.64%.
[0025] Preparation of intermediate (a1-2):
[0026] Put 2.37g a1-1 into a 100mL flask, add 40mL 2M NaOH, stir at room temperature for 14h, add 2M HCl until the solution becomes acidic, and precipitate a large amount of precipitate, filter, and dry to...
example 2
[0042] Example 2: 1-(7-hydroxy-3-1,2-benzopyrone)-N-{2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl Preparation of}-1H-1,2,3-triazole-4-carboxamide (b2)
[0043] The synthetic route of b2:
[0044]
[0045] Preparation of intermediate a2-1:
[0046] Add 2.76g of 2,4-dihydroxybenzaldehyde, 2.34g of N-acetylglycine, 4.92g of anhydrous sodium acetate, and 85mL of acetic anhydride to a 250mL flask, reflux and stir for 5h, pour the reaction solution into ice water, and precipitate a large amount of precipitate. Filter and dry to obtain 2.03 g of a yellow solid with a yield of 38.9%.
[0047] Preparation of intermediate a2-2:
[0048] Put 0.3 g of a2-1 into a 250 mL flask, add 10 mL of concentrated hydrochloric acid and 5 mL of ethanol, stir at reflux for 1 hour, cool slightly, add ice water to the reaction solution, cool the reaction solution in an ice bath, slowly add 0.24 g of NaNO 2 , after stirring for 10-15 minutes, add 0.34gNaN 3 , stirred for 1 h, filtered, washed the pr...
example 3
[0057] Example Three: 2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-{2-[4-(2-methoxyphenyl)-1-piperazine The preparation of ethyl] ethyl} thiourea} benzoic acid (b3)
[0058] The synthetic route of b3:
[0059]
[0060] Add 136mg b, 1.4mL TEA and 15mL dichloromethane to a 50mL flask, stir to dissolve, and dissolve 97mgFITC in 4mL DMF under ice bath conditions, slowly drop in, rise to room temperature, stir in the dark for 3 days, spin out Solvent, in the form of oil, add ethyl acetate and methanol, precipitate a large amount of precipitate, filter, collect the precipitate, dissolve it with 2M NaOH, filter off the insoluble matter, add 2M HCl until the solution becomes acidic, precipitate a large amount of solid, filter, dry, and get yellowish brown Solid 80mg, yield 52.3%, mp: 188-190°C.
[0061] 1 HNMR (600MHz, DMSO-d 6 )δppm: 10.21 (s, 2H), 8.28 (s, 1H), 8.03 (s, 1H), 7.76 (s, 1H), 7.17 (d, 1H), 6.93-6.95 (m, 2H), 6.85-6.91 (m, 2H), 6.60-6.67(m, 2H), 6.55-6.59(m, 4H), 3.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com