Kit, amplification method and detection method for detecting SNP sites related to tacrolimus and cyclosporine a personalized medicine
A technology of tacrolimus and kit, which is applied in the field of detection kits and amplification and detection of SNP sites related to individualized medicine of tacrolimus and cyclosporine A, can solve the problem of increased susceptibility to infection, grafts Rejection, transplanted diabetes and other problems, to avoid false positive results, reduce operation steps, and achieve good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Step 1: Preparation of whole blood cell lysate
[0056] Take 300 μl of peripheral venous blood from the subject, add 700 μl of cell lysate, invert and mix 5 times, centrifuge at 12,000 rpm for 1 minute, discard the supernatant, and place the centrifuge tube upside down on clean absorbent paper for 2 minutes to ensure that the precipitate remains in the tube , add 300 μl double distilled water, vortex and shake to form a cell lysate suspension.
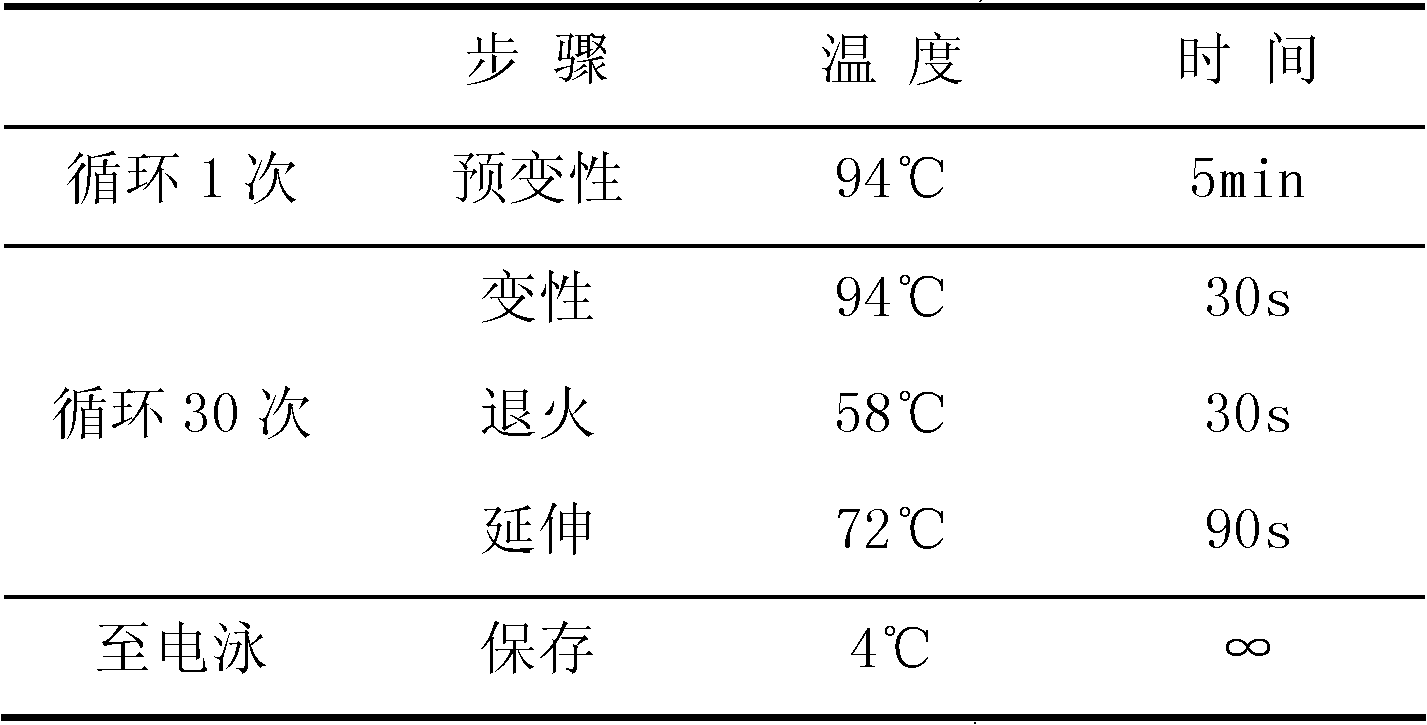
[0057] Step 2: PCR amplification reaction
[0058] 1. Configure the wild-type reaction system: Add 4.8 μl of cell lysis suspension, 5 μl of 2× wild-type amplification buffer and 0.2 μl of polymerase (2.5 U / μl) into the PCR tube to form an independent reaction system. Mix well and centrifuge briefly, see the table below for details:
[0059] 2× wild-type amplification buffer solution 5μl Cell Lysis Suspension 4.8μl Polymerase (2.5U / μl) 0.2μl
[0060] 2. Configure the mutant reaction system: add 4.8...
Embodiment 2
[0073] Step 1: Extraction and dilution of whole blood genomic DNA
[0074] Take 300 μl of peripheral venous blood from the subject, and extract whole blood genomic DNA according to the instructions of the whole blood genomic DNA extraction kit. Measure the concentration of DNA with a spectrophotometer and dilute to 15-20 ng / μl.
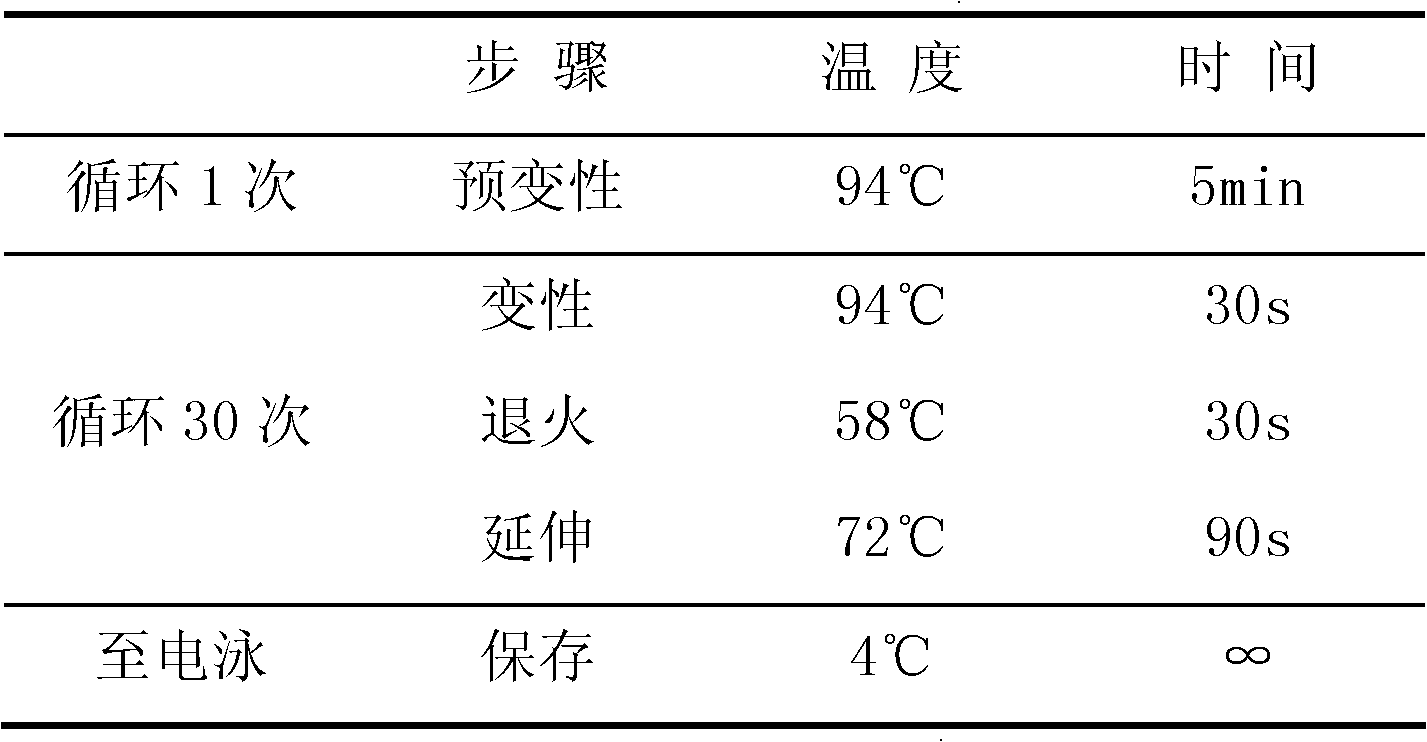
[0075] Step 2: PCR amplification reaction
[0076] 1. Configure the wild-type reaction system: add 4.8 μl of cell lysis suspension, 5 μl of 2× wild-type amplification buffer, and 0.2 μl of polymerase (2.5 U / μl) into the PCR tube to form an independent reaction system. Mix well after adding the sample. Evenly, briefly centrifuge, see the table below for details:
[0077] 2× wild-type amplification buffer solution 5μl Cell Lysis Suspension 4.8μl Polymerase (2.5U / μl) 0.2μl
[0078] 2. Configure the mutant reaction system: add 4.8 μl of cell lysis suspension, 5 μl of 2× mutant amplification buffer and 0.2 polymerase (2.5 U / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com