A kind of aip polypeptide derivative of anti-staphylococcus aureus agrc quorum sensing system and application thereof
A technology of quorum sensing system and derivatives, applied in antibacterial drugs, medical preparations containing active ingredients, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
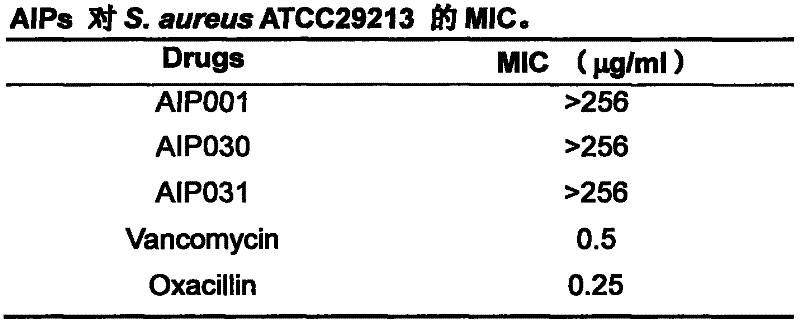
[0024] Example 1 Determination of antibacterial activity of AIPs in vitro.
[0025] The first step is to dilute the drug doubly. Weigh 2mg of the drug and add it to 1ml of the diluent and mix evenly. The concentration of the drug stock solution is 2mg / ml. After the original solution is diluted, it is sterilized by filtration, and a small amount is used for equipment. 512μl stock solution was added to 488μl M-H broth medium, and the highest concentration of antibacterial drugs after mixing was 1024μg / ml. Pipette 100 μl of the drug with the highest concentration and add it to the No. 1 hole in each row. After mixing, suck out 100 μl from the No. 1 hole, add it to No. 2, and then multiply and dilute it to No. 10, then suck out 100 μl and discard it, so that the gradient concentration of the drug is 512, 256, 128, 64, 32, 16, 8, 4, 2, 1 μg / ml.
[0026] The second step is to revive the bacteria. Take the test bacteria frozen at -20°C, streak and inoculate them on M-H agar mediu...
Embodiment 2
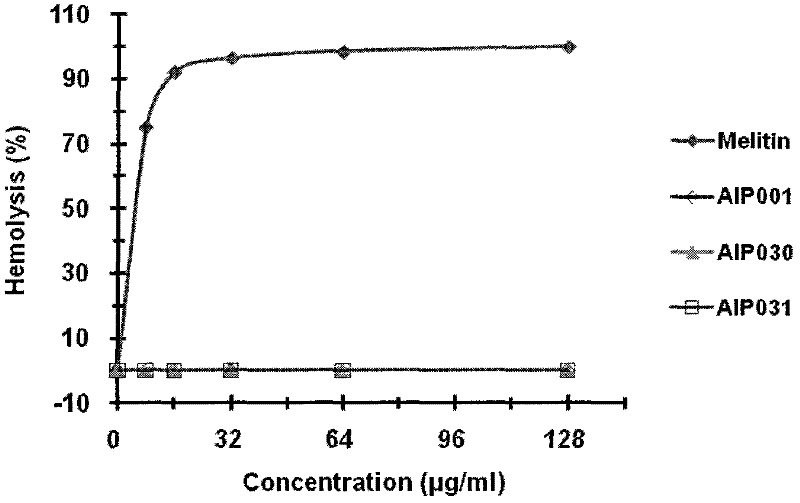
[0030] Example 2 Determination of hemolytic toxicity of AIPs.
[0031]The first step is to take anticoagulated fresh mouse peripheral blood, centrifuge at 3000r / min for 10min, absorb the blood cells in the lower layer after layering, add 0.01M PBS buffer to resuspend, wash to remove plasma and brown blood film layer, repeat 3 times Finally, they were resuspended in different volumes of 0.01M PBS to obtain 4% and 20% red blood cell suspensions.
[0032] In the second step, 100 μl of resuspended erythrocytes and 100 μl of two-fold serially diluted antimicrobial peptides (final concentration of erythrocytes is 2%) were taken and incubated in a 37° C. incubator.
[0033] In the third step, after 1 hour, the reaction solution was centrifuged, and its optical density value was measured at 540 nm.
[0034] In the fourth step, the OD of erythrocyte suspension incubated with an equal volume of 0.01M PBS 540 As 0% hemolysis, the OD incubated with an equal volume of Triton-100 (final c...
Embodiment 3
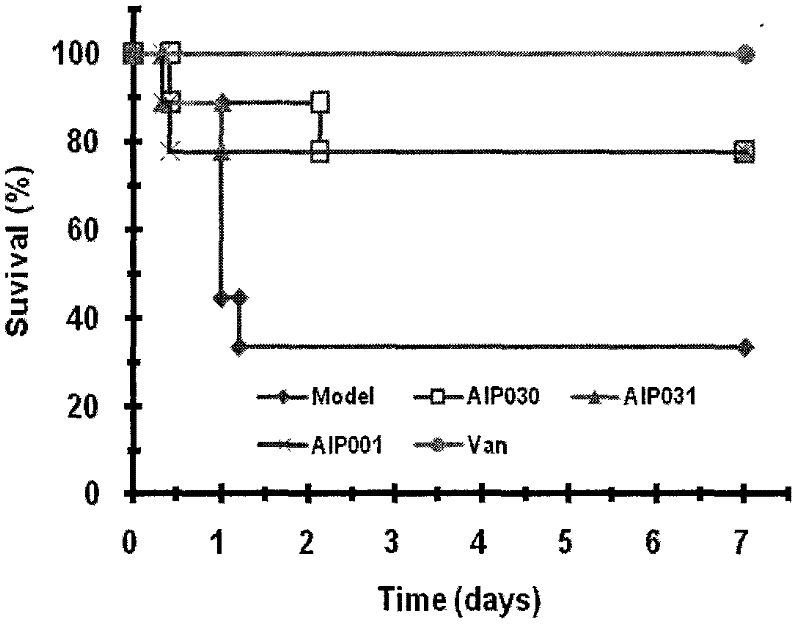
[0036] Example 3 In the BALB / c mouse Staphylococcus aureus ATCC29213 sepsis model, the determination of the survival curve of BALB / c mice.
[0037] BALB / c mice were intraperitoneally injected with a certain amount of S.aureus suspension to establish a sepsis model. One hour after the infection, the mice were given 10 mg / kg AIP030 and AIP031, and the other group was given the antibiotic norvancomycin hydrochloride After treatment, observe continuously for 7 days, and record the survival time of the mice.
[0038] The result is as image 3 As shown, AIP001 and the modified AIP derivatives AIP030 and AIP031 can significantly prolong the survival time of BALB / c mice and improve the survival rate of mice; and AIP030 is better than AIP031 and AIP001 in prolonging the survival time of mice. After 7 days, the survival rates of AIP030, AIP031 and AIP001 mice were 77.78%, 77.78% and 77.78%, respectively. It shows that AIP030, AIP031 and AIP001 can significantly improve the survival ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com