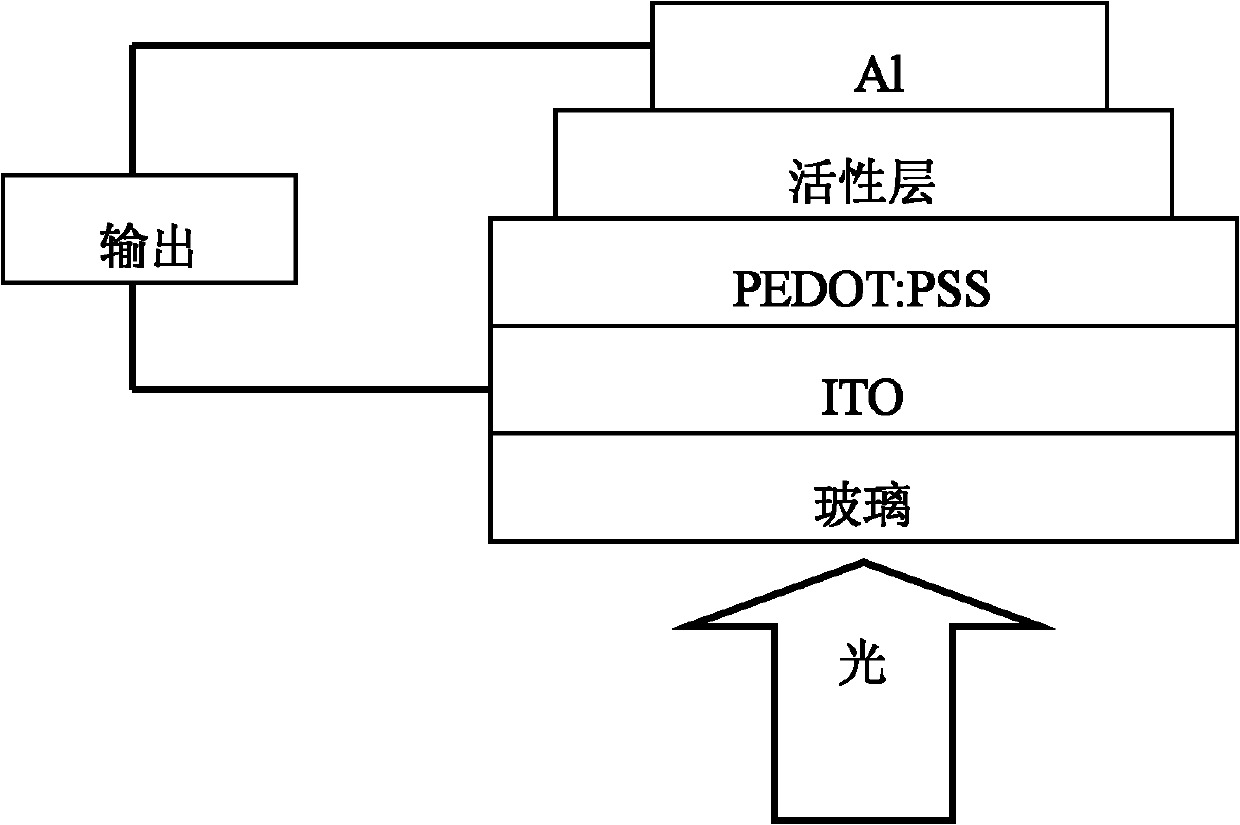
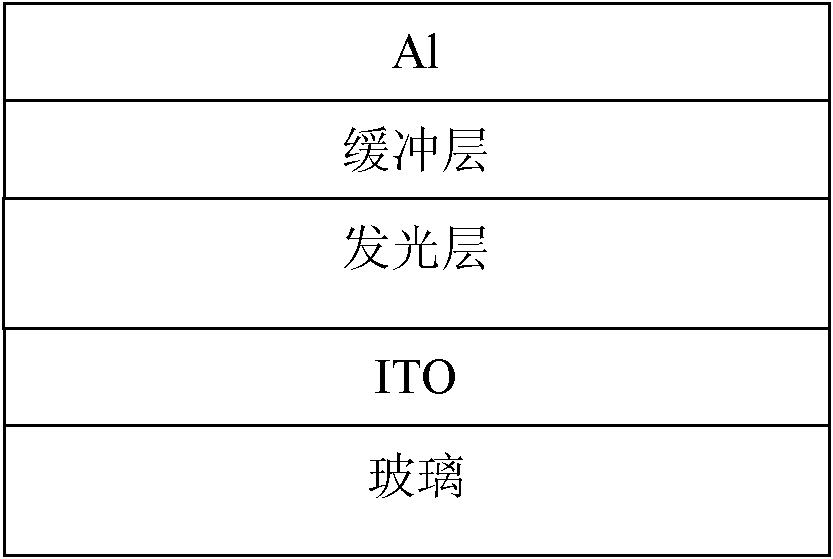
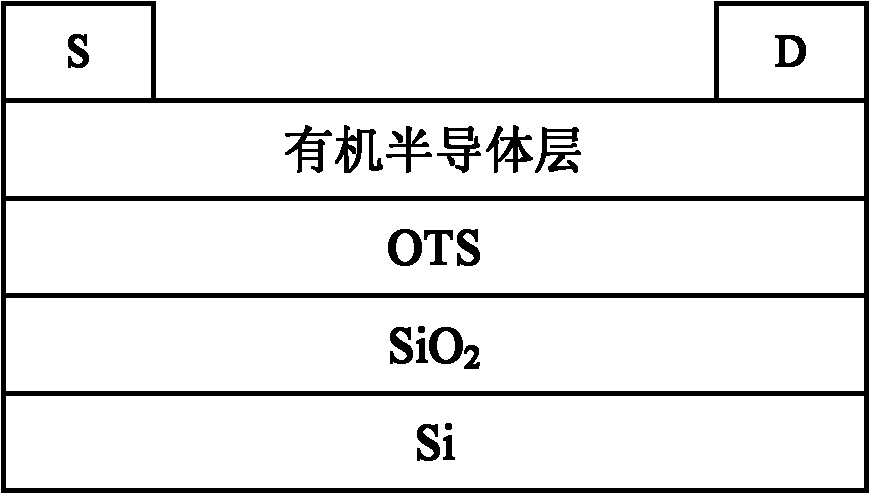
Organic semiconductor material containing fluorene, anthracene and thienothiophene units, preparation method and application thereof
A technology of organic semiconductors and thiophene units, which is applied in the field of preparation and organic semiconductor materials, can solve the problems of low photoelectric conversion efficiency of organic solar cells, achieve excellent charge transport performance, improve transport speed and efficiency, and facilitate film-forming processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 This example discloses polymers P1 and P2 with the following structures, wherein n=10 in polymer P1 and n=45 in polymer P2:
[0038]
[0039] Polymer P1 x=0.1, y=0.9
[0040] P2 x=0.5, y=0.5
[0041] The preparation steps of P1 and P2 are as follows:
[0042] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene:
[0043]
[0044] Tetrahydrofuran refining: add tetrahydrofuran solvent that has been dried with KOH for several days into a 1000ml single-necked bottle, then cut into sodium block and add benzophenone, keep stirring and reflux until the system turns dark purple, distill out the refined THF for later use.
[0045] Set up an anhydrous and anaerobic reaction device, stirring and N 2 Under the protection of the three-necked flask, add 9.0mmol of white 2,7-dibromo-9,9-dioctylfluorene, inject 150ml of the above-mentioned refined tetrahydrofuran solvent with a syringe, and then use a syringe to slowly Inj...
Embodiment 2
[0055] Example 2 This example discloses polymers P3 and P4 with the following structures, wherein n=13 in polymer P3 and n=18 in polymer P4:
[0056]
[0057] Polymer P3 x=0.8, y=0.2
[0058] P4 x=0.2, y=0.8
[0059] The preparation steps of P3 and P4 are as follows:
[0060] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene:
[0061] For the preparation process, see Step 1 in Example 1.
[0062] Step 2, preparation of P3 and P4:
[0063]
[0064] Polymer P3 x=0.8, y=0.2
[0065] P4 x=0.2, y=0.8
[0066] Add 1mmol 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, 0.8mmol 9,10-dibromo-2,6-bis(2-octyldecyl)anthracene (see Klaus Mullen et al. Macromol.Chem.Phys.2006, 207, 1107-1115 for the synthesis method of this compound), 0.2mmol2, 5-dibromothieno[3,2-b]thiophene, 0.02mmol tetrakistriphenylphosphine palladium, 10ml Na with a concentration of 2mol / L 2 CO 3 Aqueous solution and 40ml toluene s...
Embodiment 3
[0070] Example 3 This example discloses polymers P5 and P6 with the following structures, wherein n=20 in polymer P5 and n=28 in polymer P6:
[0071]
[0072] Polymer P5 x=0.2, y=0.8
[0073] P6 x=0.5, y=0.5
[0074] The preparation steps of P5 and P6 are as follows:
[0075] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene:
[0076] For the preparation process, see Step 1 in Example 1.
[0077] Step 2, preparation of P5 and P6:
[0078]
[0079] Polymer P5 x=0.2, y=0.8
[0080] P6 x=0.5, y=0.5
[0081] Add 1mmol 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene, 0.2mmol 9,10-dibromo-2,6-bis(2-octyldecyl)anthracene, 0.8mmol 3,6-dihexyl-2,5-dibromothieno[3,2-b]thiophene (the compound The synthesis of see document He, Y.; Wu, W.; Macromolecules 2008,41,9760 of Zhao, G. etc.), 0.022mmol tetrakistriphenylphosphine palladium, 10ml concentration is the Na of 2mol / L 2 CO 3 Aqueous solution and 40ml t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com