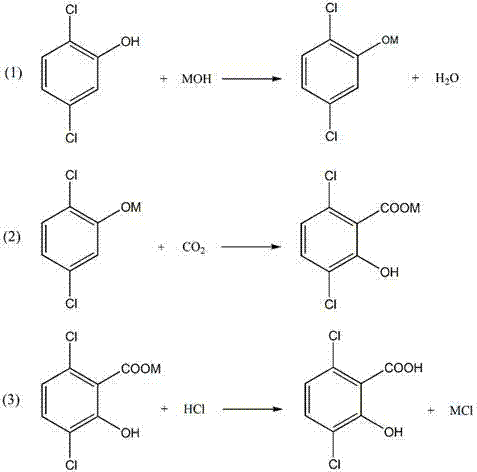
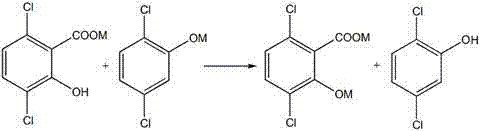
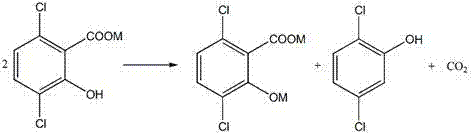
A method for increasing the yield of 3,6-dichloro-2-hydroxybenzoic acid
A technology of hydroxybenzoic acid and dichlorophenol, applied in the directions of carboxylate preparation, organic chemistry, etc., can solve the problems of not taking into account phenol, long reaction time, unsatisfactory effect, etc., reducing the reaction time and improving the yield per pass , the effect of saving the reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh 86g of 2,5-dichlorophenol (95%) in a 1000ml four-neck flask and dissolve in 34.3g of KOH (90%) and 28.27ml of H 2 O, then add 500ml of xylene as a solvent and mix evenly, heat up and dehydrate until almost no water is evaporated, take the potassium phenate suspension to measure the water, when the water is lower than 0.1%, it can be transferred to the autoclave, and the high-pressure reaction is turned on The inlet valve of the kettle is fed into CO 2 Remove the air in the autoclave, continue to ventilate for 10 minutes, and then heat the autoclave to raise the temperature, start stirring at the same time, and pass CO into the autoclave. 2 , Carry out a carboxylation reaction, the reaction temperature and pressure are controlled at 160°C and 8 MPa, and the reaction time is 4hr. After the carboxylation is completed, feed cooling water into the cooling pipe of the autoclave. When the system cools down to 100°C, add 50g of 40% KOH aqueous solution into...
Embodiment 2
[0039] Take 405g (95%) of 2,5-dichlorophenol, add KOH aqueous solution (176g KOH, 180ml H2O), and dehydrate in 500ml xylene solvent, the steps are the same as in Example 1. Then transfer to an autoclave for a carboxylation reaction, the reaction temperature is 140°C, the pressure is 6MPa, and the reaction time is 6h. After cooling, add KOH aqueous solution (90gKOH, 90mlH 2 O) carry out azeotropic dehydration, then add 70gNa 2 CO 3 The carboxylation reaction was carried out, and then CO was continuously introduced into the 2 Carry out the second carboxylation in 10 minutes, the second carboxylation conditions are also 140°C, 6MPa, and the reaction time is 6hr. After the secondary carboxylation reaction, 300ml of cooling water was introduced to reduce the temperature of the system to 100°C, stirred evenly, and the water phase was taken after layering, and then an aqueous hydrochloric acid solution with a mass fraction of 30% was added dropwise to the water p...
Embodiment 3
[0041] Weigh 86g of 2,5-dichlorophenol (95%) in a 1000ml four-necked flask and dissolve it in 34g of KOH (90%) and 25.5ml of H 2 In O, heat up and dehydrate in 300ml toluene until almost no water is evaporated, take a phenoxide suspension sample to measure the moisture, when the moisture is lower than 0.1%, it can be transferred to the autoclave, and the inlet valve of the autoclave is opened. Into CO 2 Remove the air in the autoclave, continue to ventilate for 10 minutes, and then heat the autoclave to raise the temperature, start stirring at the same time, and pass CO into the autoclave. 2 , Carry out a carboxylation reaction, the reaction temperature and pressure are controlled at 140°C and 6 MPa, and the reaction time is 6hr. After the carboxylation is completed, feed cooling water into the autoclave cooling pipe, then add 70g40% KOH aqueous solution in the still, and react while stirring until the dichlorophenol by-product is all converted into phenate. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com