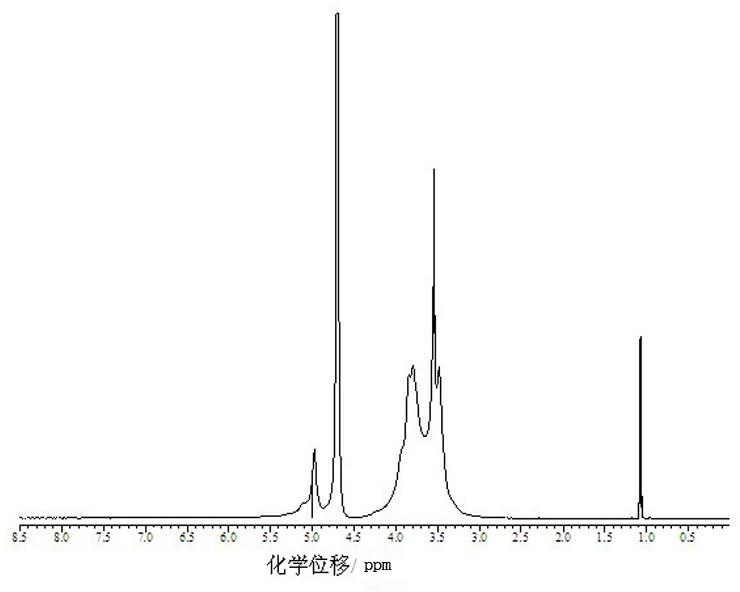
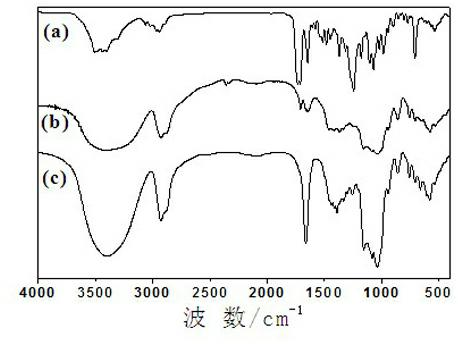
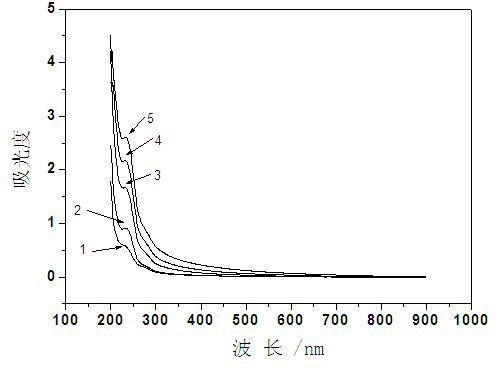
Preparation method for beta-cyclodextrin polymer-paclitaxel inclusion compound
A technology of cyclodextrin polymer and paclitaxel, which is applied in the directions of non-active ingredient medical preparations, drug combinations, pharmaceutical formulations, etc., can solve the problems of difficult inclusion reaction, influence of drug concentration, waste of original drug paclitaxel, etc. Ease of industrial production, promotion of effective collision, excellent water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be described in detail below in conjunction with examples.
[0026] 1. Preparation of β-cyclodextrin polymer:
[0027] Add 19.7 g of sodium hydroxide, 25 g of β-cyclodextrin and 40 mL of water into a 250 mL three-neck flask, stir at room temperature until the solids are completely dissolved, then add 6-12 mL of epichlorohydrin, heat in a water bath at 30°C, and stir for 24 h.
[0028] After the reaction was completed and the temperature of the reaction system dropped to room temperature, the solution was dialyzed until the pH value of the solution was neutral. The solution was subjected to rotary evaporation until it became viscous, and absolute ethanol was added to precipitate a white solid, which was filtered and vacuum-dried at 50°C for 24 hours. Finally, a white β-cyclodextrin polymer is obtained with a yield of 50-60%.
[0029] The molecula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com