Medicinal composition and preparation method thereof
A composition and drug technology, which is applied in the direction of drug combination, pharmaceutical formula, non-active ingredients of polymer compounds, etc., can solve the problems of poor water solubility of drugs, achieve the effects of convenient operation, good solubility, and improved utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
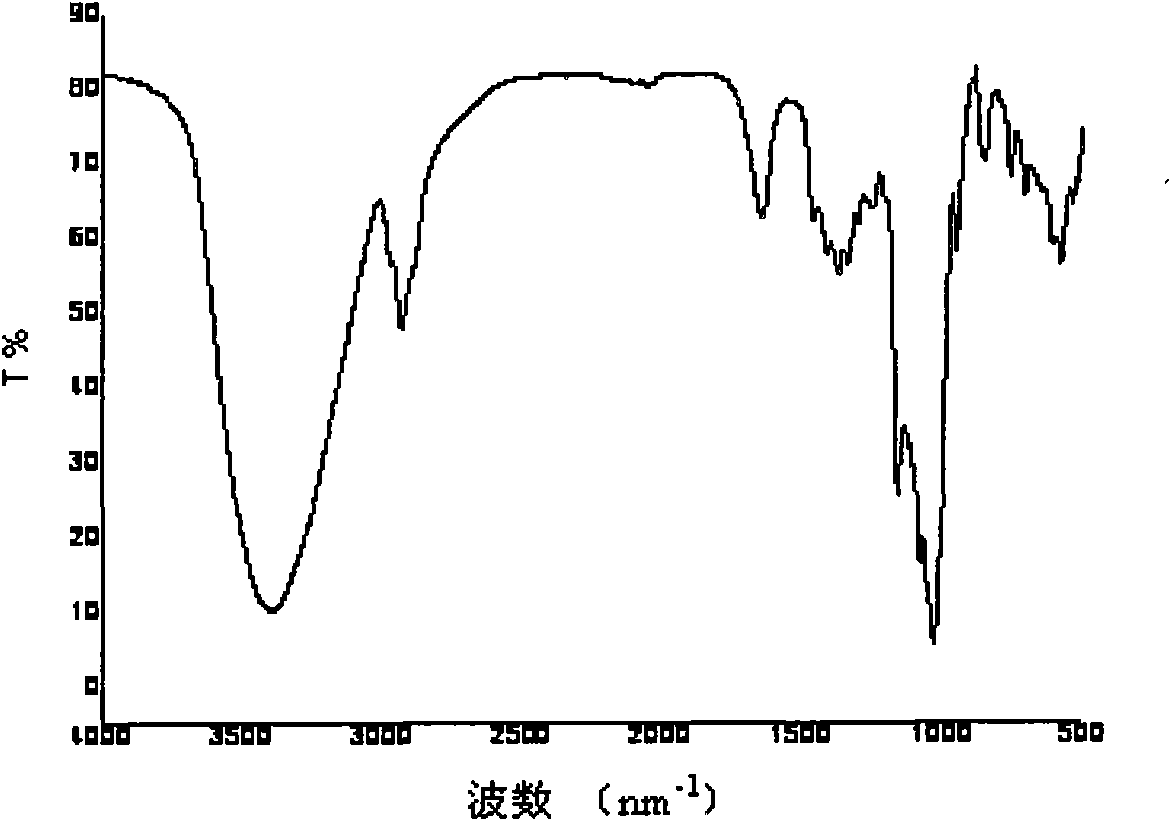
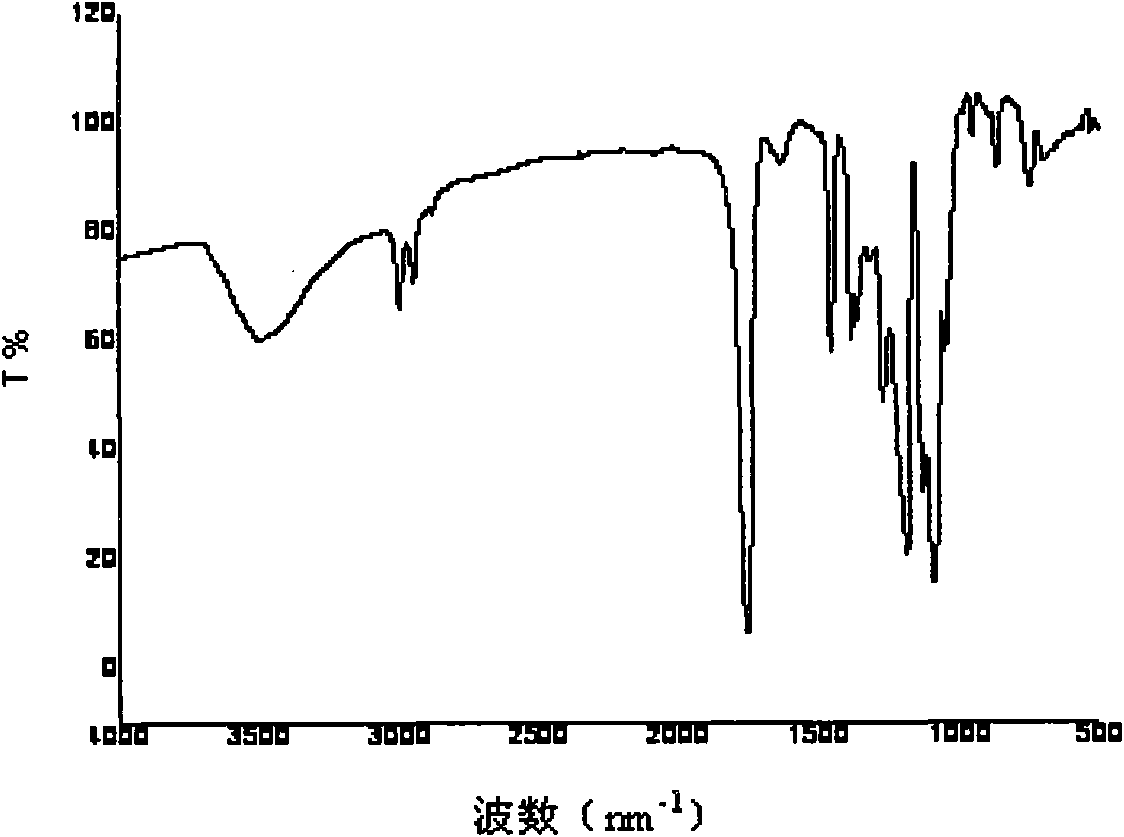
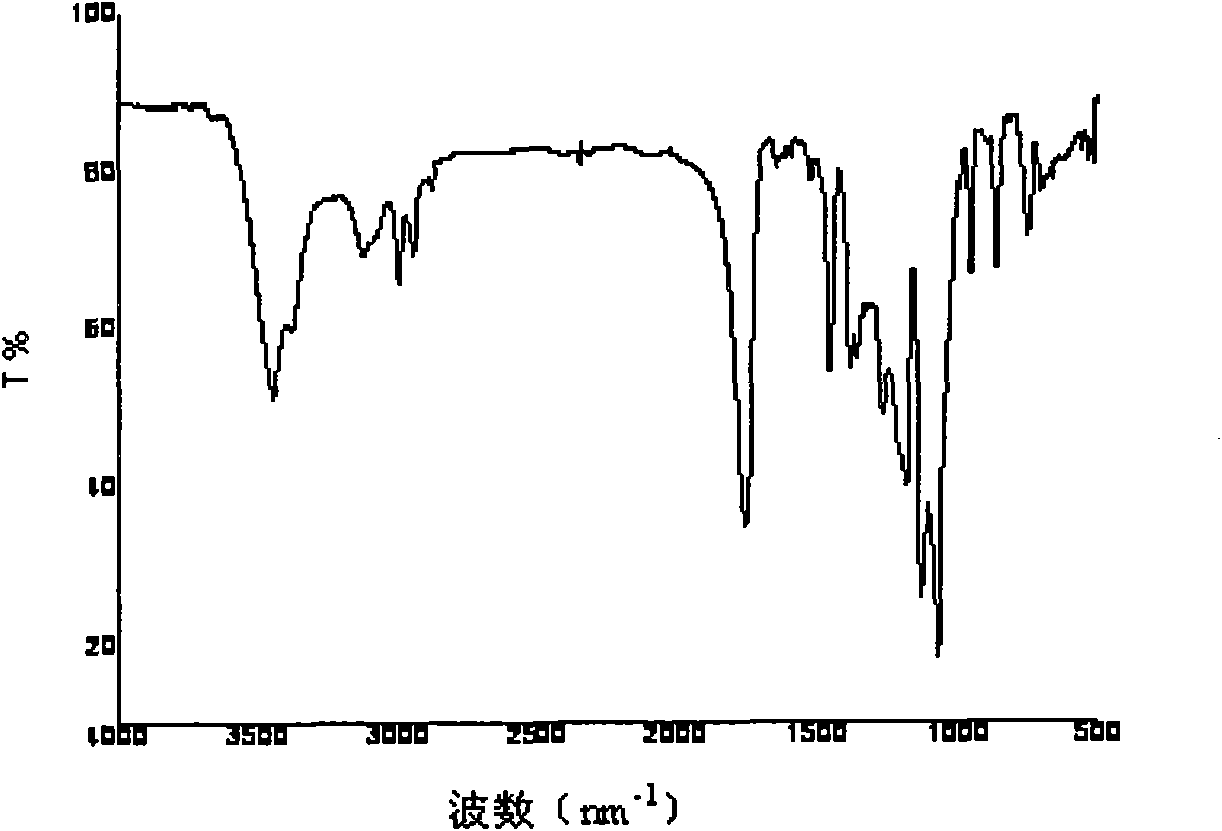
Image
Examples
preparation example Construction
[0046] According to the preparation method provided by the present invention, the contacting in step (a) is carried out under an inert atmosphere, and the contacting conditions include: the molar ratio of the ester to the cyclodextrin is 1-15:1, relatively For 1g of cyclodextrin, the amount of the organic amine is 0.01-5ml, the amount of the first organic solvent is 4-100ml, the contact temperature is 70-85°C, and the contact time is 10-15 hours.
[0047] Since lactide, glycolide and caprolactone are ring-opened under the action of the catalyst, they can directly react with -OH on the cyclodextrin. Therefore, in the present invention, one or both of lactide, glycolide and caprolactone are selected to be directly contacted with cyclodextrin to obtain the cyclodextrin-aliphatic polyester compound represented by formula (6). branch polymer. Lactide, glycolide, and caprolactone used in the present invention are all commercially available.
[0048] According to the preparation me...
Embodiment 1
[0068] Preparation of hydroxypropyl β-cyclodextrin-polylactic acid-dipalmitoylphosphatidylethanolamine grafted polymer nanoparticles loaded with paclitaxel
[0069](1) 5.64g of lactide (Alfar Aesar company, 97%, analytically pure) and 2.5g of hydroxypropyl β-cyclodextrin (Japan Sigma-Aldrich company) are placed in a three-necked flask and vacuumized for 1 hour Finally, add 10 mL of distilled dimethyl sulfoxide to dissolve, add 0.5 mL of triethylamine (Xilong Chemical Factory, Shantou City, Guangdong, analytically pure), and react at 80 ° C for 12 hours under nitrogen protection to obtain hydroxypropyl β - Cyclodextrin-polylactic acid graft polymer crude product. Then use 200mL water to precipitate, wash with water (100mL×4 times), dry in a vacuum oven at 20°C for 48 hours, then extract with toluene (20mL×2 times), and dry in a vacuum oven at 20°C for 48 hours. 6.11 g of solid product hydroxypropyl β-cyclodextrin-polylactic acid graft polymer was obtained. Calculated n=14.
...
Embodiment 2
[0086] Preparation of hydroxypropyl β-cyclodextrin-polylactic acid-dipalmitoylphosphatidylethanolamine grafted polymer nanoparticles loaded with paclitaxel
[0087] Steps (1) to (3) are carried out according to the method of Example 1, and the amounts, conditions and results of each substance involved in the method are shown in Table 1 to Table 3.
[0088](4) 20 mg of purified hydroxypropyl β-cyclodextrin-polylactic acid-dipalmitoylphosphatidylethanolamine graft polymer was dissolved in 3 mL of dichloromethane to obtain cyclodextrin-aliphatic polyester-phosphatidyl Ethanolamine grafted polymer solution, 0.2mg paclitaxel (Norui Pharmaceutical Technology Co., Ltd., Beijing, lot number: 090328) was dissolved in 0.2mL dichloromethane to obtain a paclitaxel solution, cyclodextrin-aliphatic polyester-phosphatidylethanolamine Graft polymer solution and paclitaxel solution were mixed, and the mixed solution was added to 20mL aqueous solution containing 0.4%wt surfactant polyvinyl alco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com