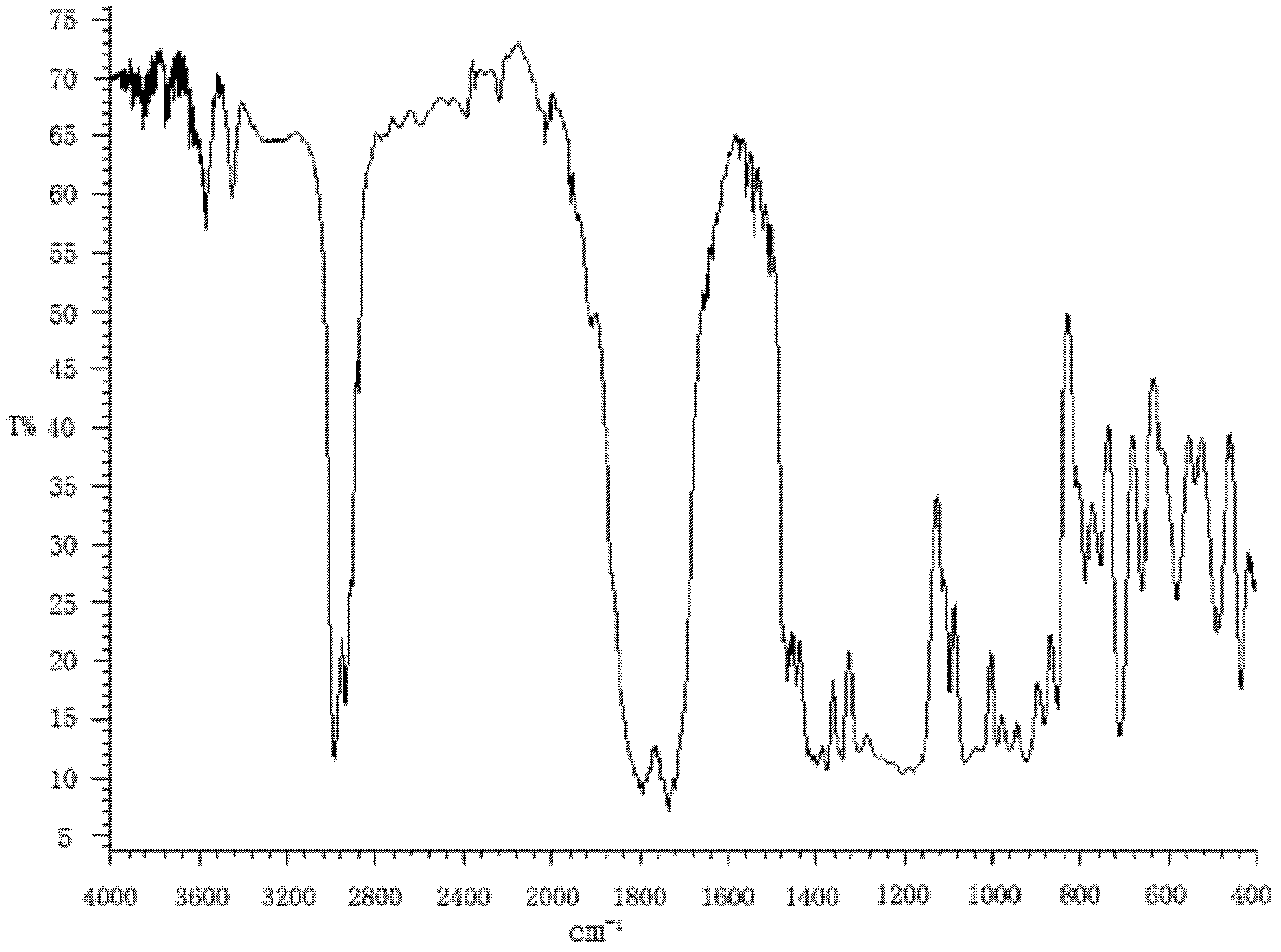
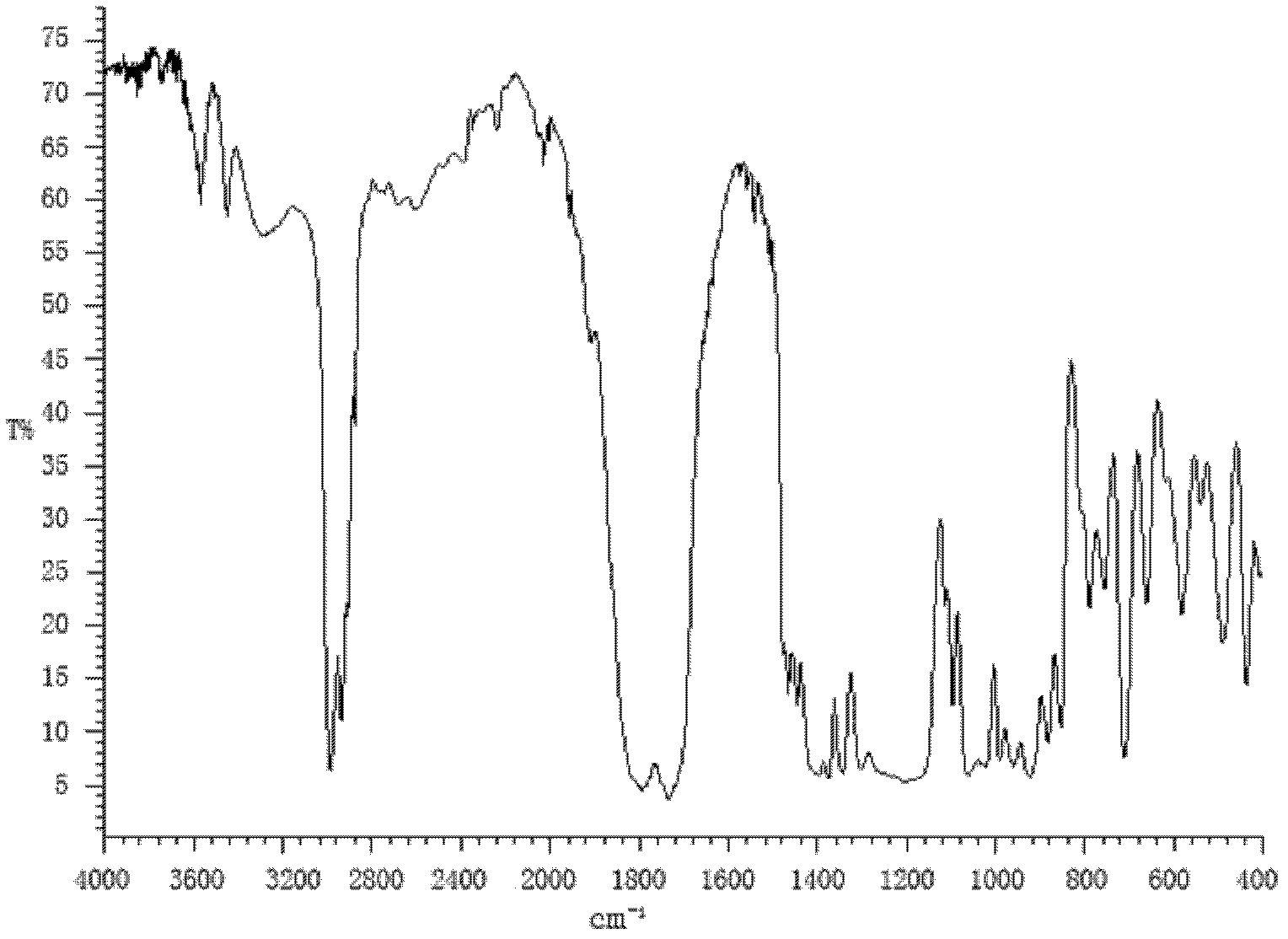
Preparation method for succinic acid monoethyl ester acyl chloride
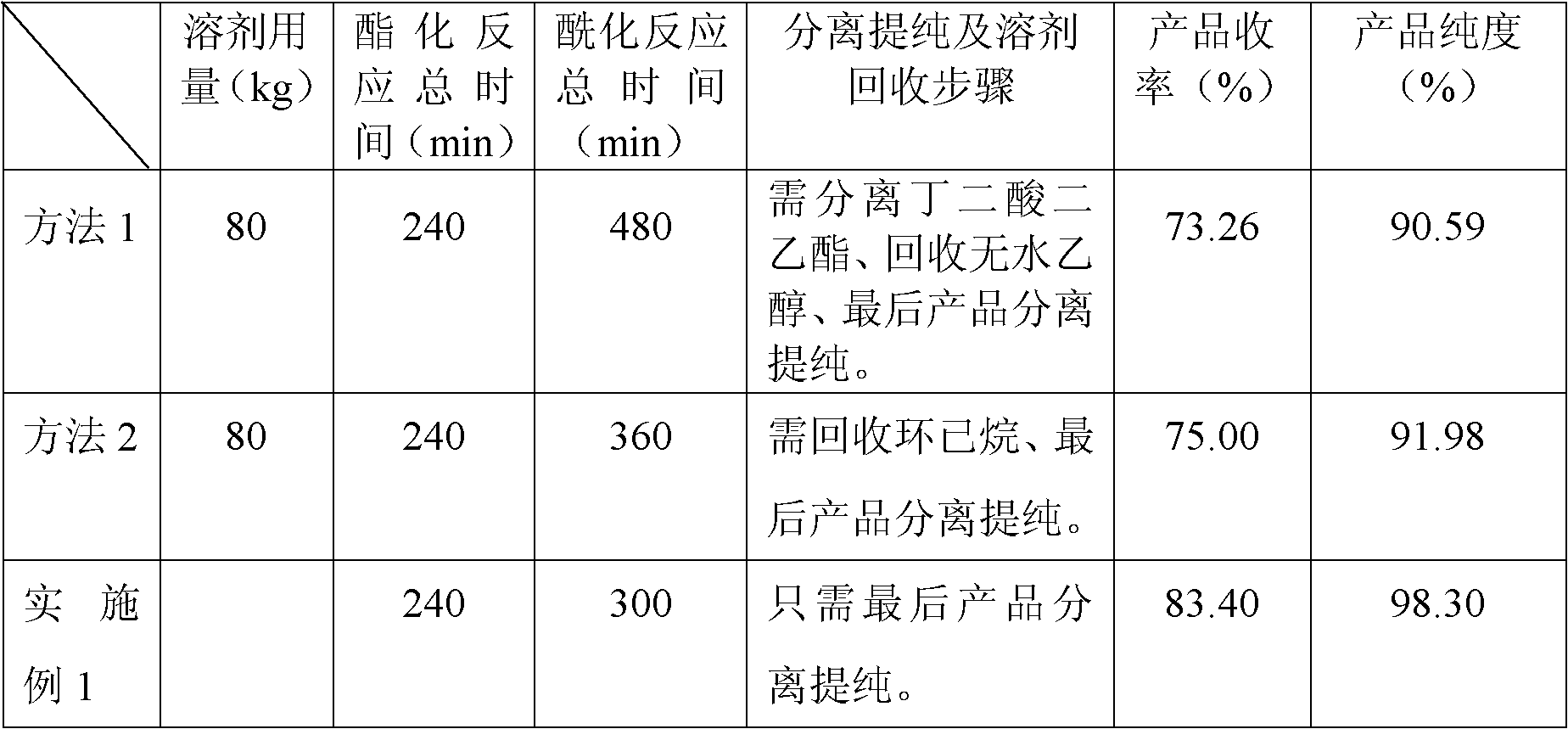
A technology for monoethyl succinate acid chloride and crude monoethyl ester acid chloride, which is applied in the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc. The problems of low purity and high production cost can save the solvent separation and recovery process, improve the reaction rate and improve the utilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Put 50kg of absolute ethanol into the esterification reactor for the first time, then put 160kg of succinic anhydride into it at a stirring speed of 82r / m, continue stirring, heat up to 76°C and keep warm for esterification reaction, and the reaction time is 30 minutes. Pump another 40kg of absolute ethanol to the dripping tank, and drop it all into the above-mentioned esterification reaction kettle at a rate of 20kg / h at a temperature of 75-78°C to continue the esterification reaction. After the addition is completed, Then, under the condition of 75-78° C., reflux and heat preservation were carried out for esterification reaction for 90 minutes to obtain monoethyl succinate with a content of 98.35%.
[0023] Monoethyl succinate is transferred to the acylation reactor, 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxylic acid chloride 4kg is added thereto, and thionyl chloride 300kg is extracted in In the dropping tank, add thionyl chloride dropwise at a rate of 100...
Embodiment 2~3
[0031] Keep the feed intake of succinic anhydride 160kg, thionyl chloride 300kg, and catalyst 4kg constant, change the feed intake of dehydrated alcohol, add dehydrated ethanol dropwise for the second time and add dehydrated alcohol mass ratio 1: 1.25 for the first time. Change, other operating conditions and product characterization methods are all the same as in Example 1 to obtain monoethyl succinic acid chloride. The dosage and test results of absolute ethanol are shown in Table 2.
[0032] Table 2. The feeding amount and test result of embodiment 2~3 dehydrated alcohol
[0033]
Embodiment 4~5
[0035] Keep 160kg of succinic anhydride, 90kg of absolute ethanol total amount (the second time is 1: 1.25 for the first time mass ratio), the feed intake of catalyst 4kg is constant, change the feed intake of thionyl chloride, other operating conditions and products The characterization methods were all the same as in Example 1 to obtain monoethyl succinic acid chloride. The dosage and test results of thionyl chloride are shown in Table 3.
[0036] Table 3. The feeding amount and test result of embodiment 4~5 thionyl chloride
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com