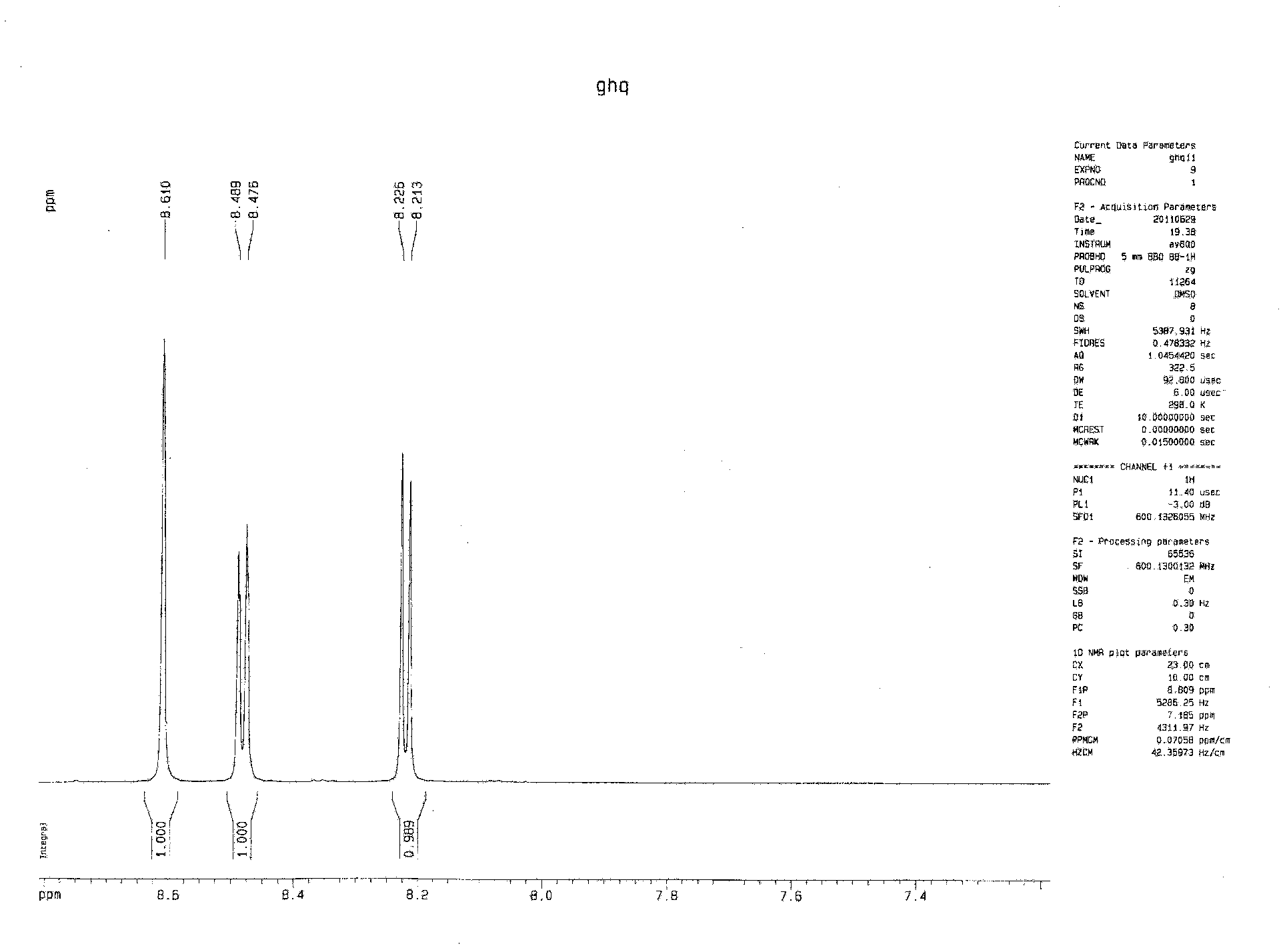
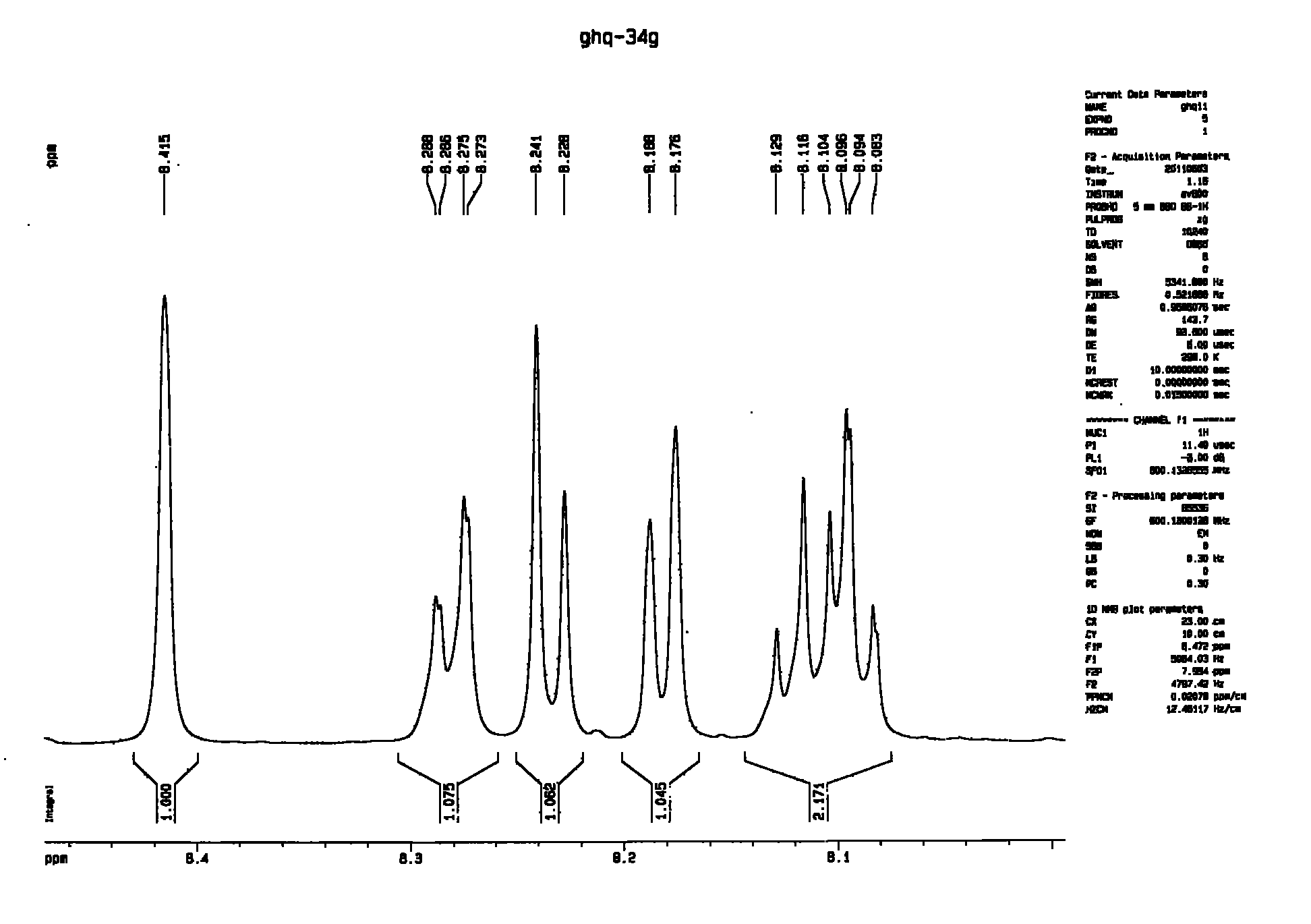
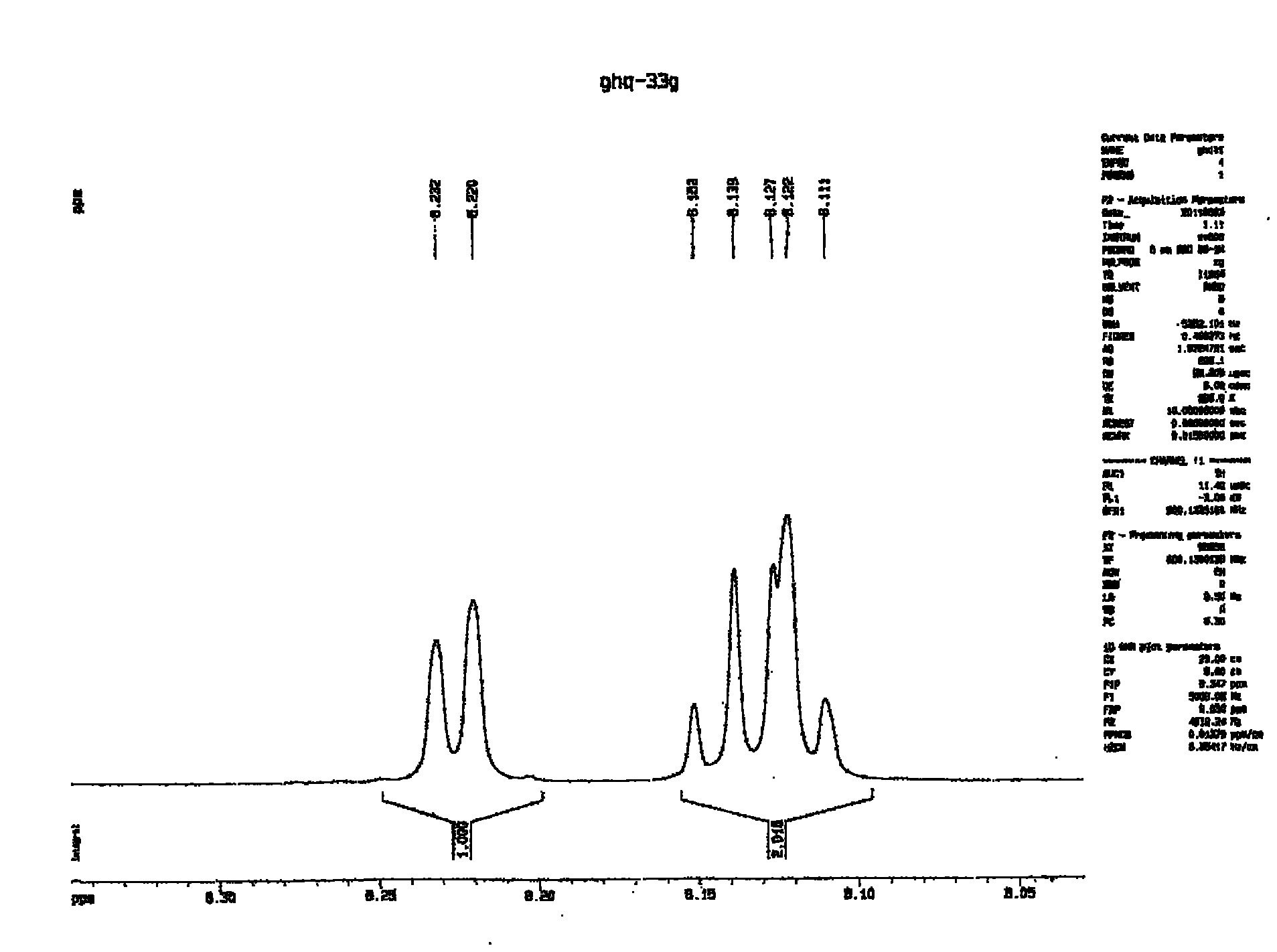
Method for preparing biphenyltetracarboxylic dianhydride (BPDA)
A technology of biphenyl dianhydride and tetramethylbiphenyl, which is applied in the field of biphenyl dianhydride preparation and can solve problems such as high cost and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention discloses a preparation method of biphenyl dianhydride, comprising the following steps:
[0024] Using acetic acid as a solvent, a cobalt source compound as a catalyst, and a bromine source compound as a cocatalyst, reacting tetramethylbiphenyl with a gas containing molecular oxygen to obtain biphenyltetracarboxylic acid;
[0025] The biphenyl tetracarboxylic acid is dehydrated to obtain biphenyl dianhydride.
[0026] In the present invention, the cobalt source compound is preferably cobalt acetate and its hydrate, cobalt naphthenate and its hydrate, cobalt sulfate and its hydrate, cobalt acetylacetonate and its hydrate, cobalt benzoylacetonate and its hydrate Cobalt bromide and its hydrate, cobalt carbonate and its hydrate, cobalt chloride and its hydrate, cobalt fluoride and its hydrate, cobalt nitrate and its hydrate, cobalt stearate and its hydrate One or more, more preferably cobalt acetate. The bromine source compound is preferably bromine, hydroge...
Embodiment 1
[0054] At room temperature, add 0.5mol 4-chloro-o-xylene (70.3g), 0.025mol nickel bromide (5.46g), 0.05mol triphenylphosphine (13.1g), 0.6mol zinc powder (39.2 g), 0.025mol 2,2'-bipyridine (3.90g) and 150ml dimethylacetamide were reacted at 70° C. for 6 hours, then added toluene, filtered to remove solid residue, and after the toluene layer was washed twice, Concentrate to remove the solvent, slowly add methanol to the solvent-removed substance, and let it stand for 24 hours to obtain a white solid; filter the white solid and dry it in vacuo to obtain 44.6 g of 3,4,3',4'-tetramethyl Base biphenyl, the yield is 85%.
[0055] Add 0.1mol 3,4,3',4'-tetramethylbiphenyl (21.03g), 0.001mol cobalt acetate (0.177g), 0.0005mol sodium bromide (0.051g) and 100g acetic acid, in described reaction vessel, feed air, make pressure in the vessel remain on 0.5MPa;
[0056] Heat the reaction vessel so that the temperature in the reaction vessel reaches 100°C, and continue the reaction for 6 ho...
Embodiment 2
[0059] At room temperature, add 0.25mol 3-chloro-o-xylene (35.15g), 0.25mol 4-chloro-o-xylene (35.15g), 0.025mol nickel bromide (5.46g), 0.05mol tris Phenylphosphine (13.1g), 0.6mol zinc powder (39.2g), 0.025mol 2,2'-bipyridine (3.90g) and 150ml dimethylacetamide were reacted at 70°C for 6 hours, then added toluene, filtered Remove the solid residue, wash the toluene layer twice with water, concentrate to remove the solvent, slowly add methanol to the solvent-removed substance, and let it stand for 24 hours to obtain a white solid; filter the white solid and recrystallize it with methanol 30.55 g of 2,3,3',4'-tetramethylbiphenyl was obtained with a yield of 58.2%.
[0060] Add 0.1mol 2,3,3',4'-tetramethylbiphenyl (21.03g), 0.001mol cobalt acetate (0.177g), 0.0005mol sodium bromide (0.051g) and 100g Acetic acid, feeds air into described reaction vessel, makes pressure in the vessel remain on 0.5MPa;
[0061] The reaction vessel was heated so that the temperature in the reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com