Method for efficient expression, renaturation and purification of lipocalin
A protein and soluble technology, applied in the fields of high-efficiency expression, purification and renaturation of lipocalin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The method for preparing soluble lipocalin of the present invention comprises the following steps:
[0042] (1) denature the inclusion body of lipocalin, and separate the supernatant containing denatured lipocalin;
[0043] (2) Adjust the pH value of the obtained supernatant to 5-6, and perform renaturation with PEG with a degree of polymerization of 200-1000 and polyhydric alcohols of C2-C8 to obtain soluble lipocalin.
[0044] protein expression
[0045] The present inventors tried to express lipocalin by adopting various expression systems (such as yeast system and Escherichia coli system), and found that the Escherichia coli system is more suitable for expressing lipocalin and obtaining the protein in the form of inclusion bodies.
[0046]Studies have shown that the main reason for the formation of inclusion bodies is that the protein expression rate is too fast, so that the protein does not have enough time to fold correctly; in addition, because the cytoplasmic e...
Embodiment 1
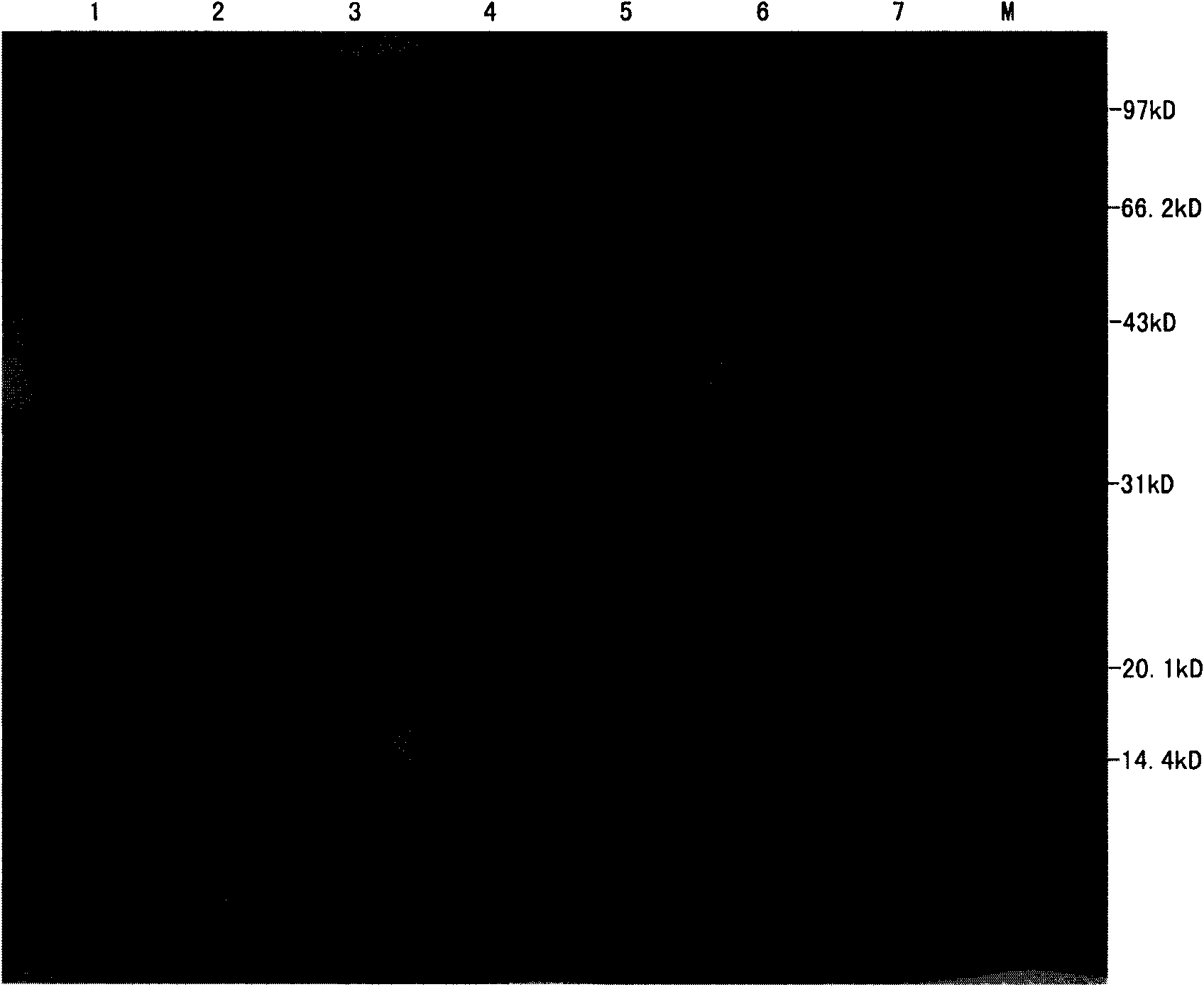
[0094] Example 1. Expression of rLcn 8 in Escherichia coli BL21 (DE3)
[0095] The expression plasmid pET28b-L was transformed into BL21(DE3) competent cells, and positive transformants were obtained by colony PCR and sequence determination. The transformants were cultured with shaking at 37°C in LB containing 50 μg / ml kanamycin. When the body density OD600 was 0.5, 1 mmol / L IPTG was added to induce the expression of the target protein.
[0096] After 5h, the bacteria were collected by centrifugation at 7000g for 5min, and the bacteria were crushed with 50ml of 50mmol / L Tris-HCl pH 8.0, 100mmol / L NaCl, 5% (v / v) glycerol mixture, and after centrifugation at 12000rpm for 20min, the precipitate and the above Clear, 15% SDS-PAGE analysis of expression.
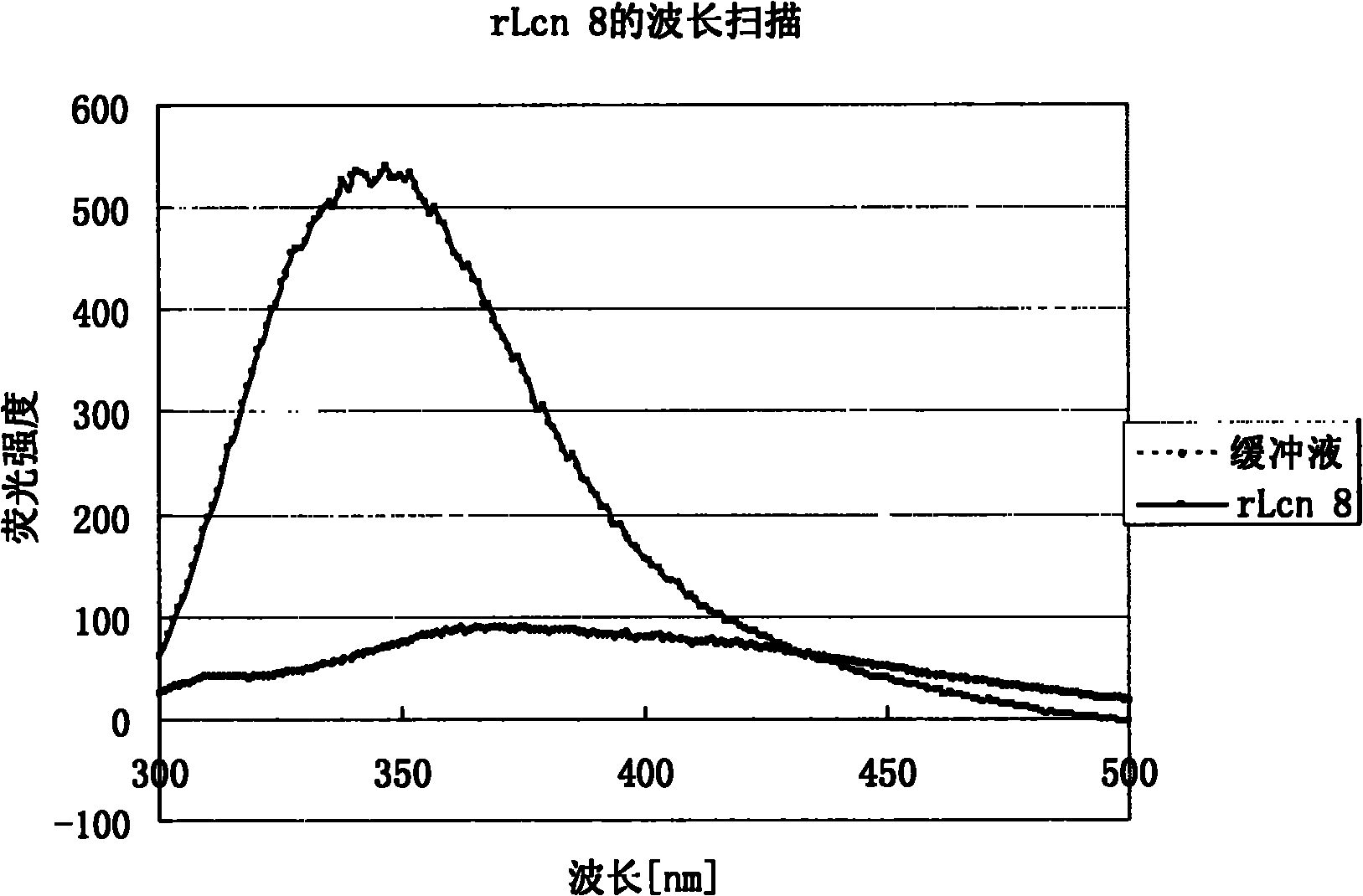
[0097] The rLcn 8 constructed in this study has a total of 182 amino acids and a theoretical molecular weight of 20847.3D. From the electrophoresis results ( figure 1 ), the rLcn 8 protein band was located near 20.1kD, which was...
Embodiment 2
[0098] Example 2. Washing and denaturation of inclusion bodies
[0099] Resuspend the precipitate obtained above with 1 mol / L urea, homogenize with a homogenizer, supplement with short-term ultrasonic pulverization, centrifuge at 12,000 rpm for 10 min, discard the supernatant, and repeat the above process once.
[0100] Inclusion body with 50mmol / L NaH 2 PO 4 , 5mol / L guanidine hydrochloride, mixed overnight at 4°C, and centrifuged at 12000rpm for 30min to remove the precipitate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com