Fluorescent material and preparation method thereof
A fluorescent material and fluorescent powder technology, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of low luminous efficiency and weak luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
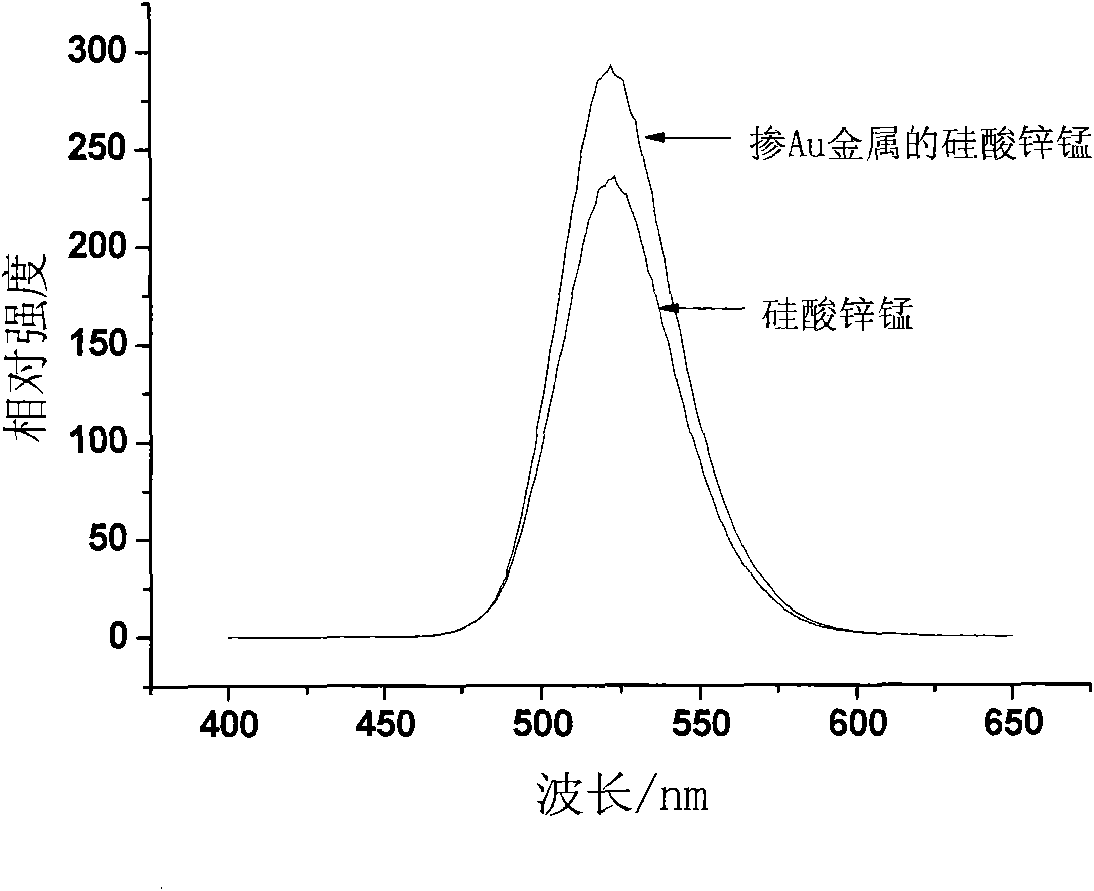
[0022] The preparation process of the fluorescent material doped with metal nanoparticles includes the following steps:
[0023] 1. Preparation of sol containing metal nanoparticles:
[0024] 1) With water or ethanol as solvent, silver nitrate (AgNO 3 ), chloroauric acid (HAuCl 4 4H 2 O), chloroplatinic acid (H 2 PtCl 6 ·6H 2 O) or palladium chloride (PdCl 2 2H 2 O) is a solute, and is prepared with an oxidizing agent solution;
[0025] 2) using water or absolute ethanol as a solvent, and at least one of hydrazine hydrate, ascorbic acid, and sodium borohydride as a solute, to prepare a reducing agent solution;
[0026] 3) Under the state of magnetic stirring, one or more additives that play a stable dispersion role are dissolved in the above-mentioned 1) oxidant solution, and the content of the additives in the finally obtained metal nanoparticle sol is 5×10 -4 g / mL~4×10 -3 g / mL; preferred additives are polyvinylpyrrolidone (PVP), sodium citrate, cetyltrimethylammon...
Embodiment 1
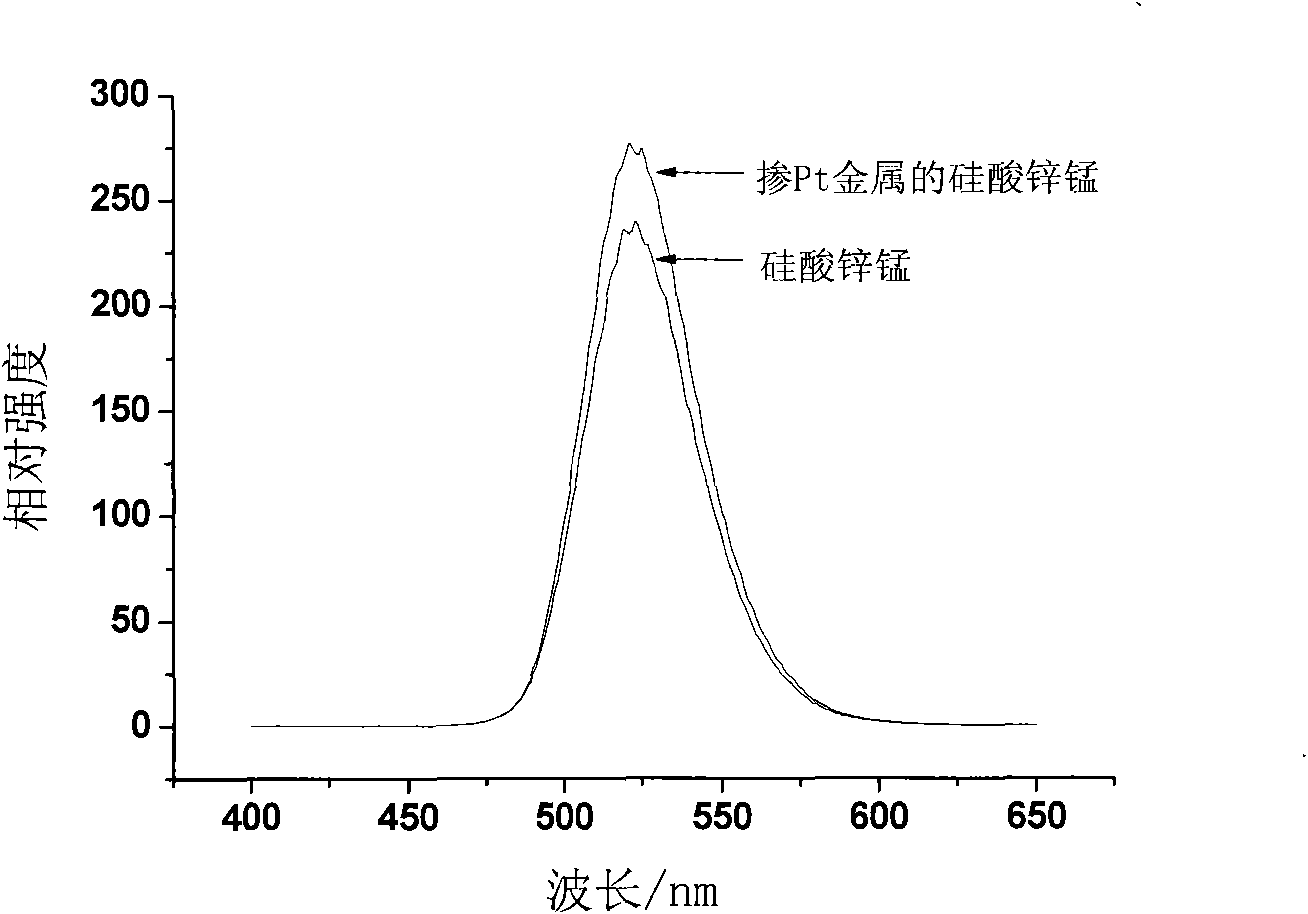
[0037] 1. Preparation of Pt nanoparticle sol
[0038] Weigh 5.18mg chloroplatinic acid (H 2 PtCl 6 ·6H 2 O) be dissolved in the deionized water of 15.2mL; After chloroplatinic acid dissolves completely, take by weighing 8.0mg sodium citrate and 12.0mg sodium dodecylsulfonate, and dissolve into chloroplatinic acid aqueous solution under the environment of magnetic stirring Medium; Weigh 3.8mg of sodium borohydride and dissolve it in 10mL of deionized water to obtain a concentration of 1×10 in 10mL -2 mol / L sodium borohydride aqueous solution; under the environment of magnetic stirring, the ratio of the amount of the reducing agent to the metal ion substance is 4.8:1, add 4.8mL sodium borohydride aqueous solution dropwise to the chloroplatinic acid aqueous solution, and then continue Reaction for 45min, the content of 20mL Pt is 5×10 -4 mol / L Pt nanoparticle sol.
[0039] 2. Preparation of fluorescent materials doped with Pt nanoparticles
[0040] 1) Fully mix 3.6mL of dis...
Embodiment 2
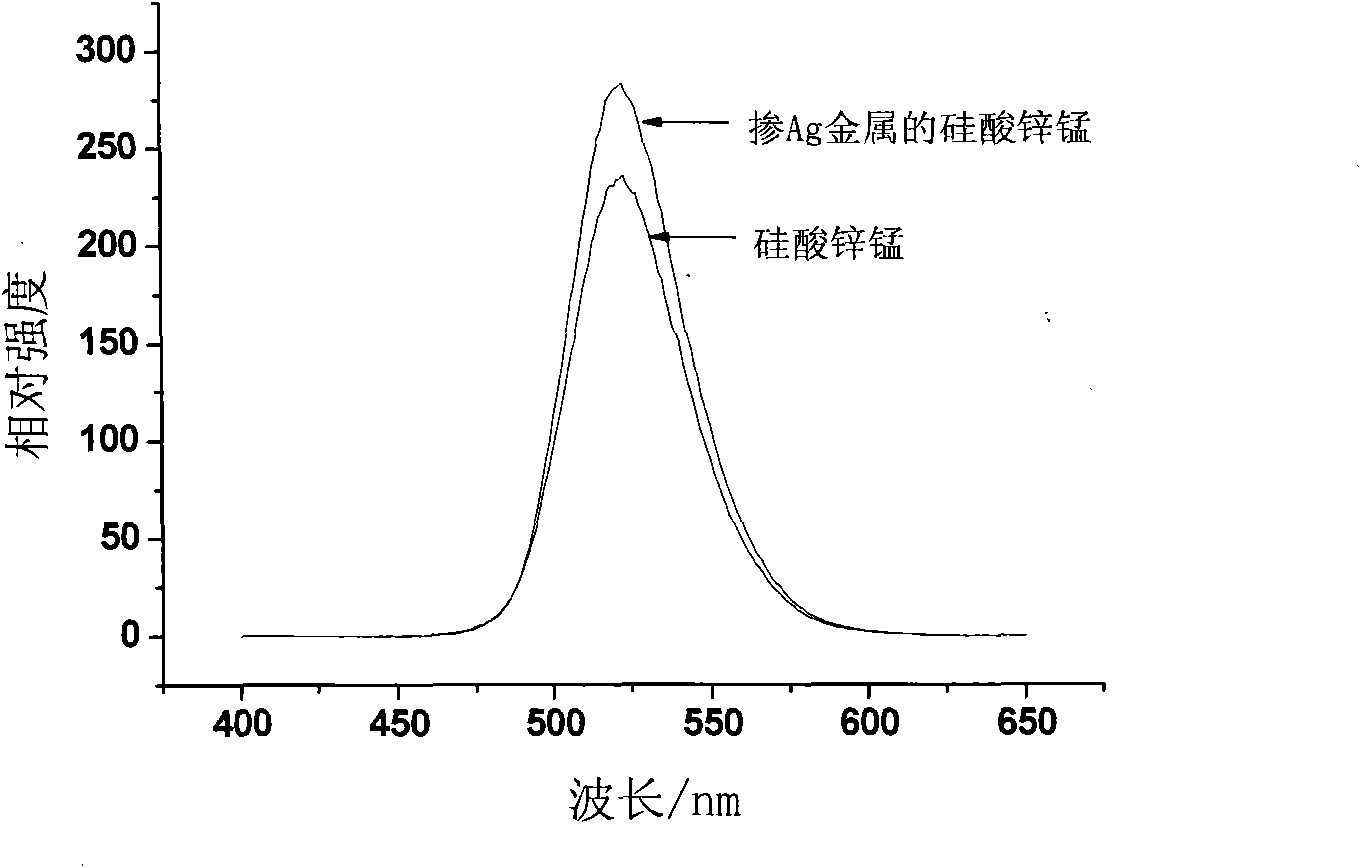
[0043] 1. Preparation of Ag nanoparticles sol
[0044] Weigh 17.0mg silver nitrate (AgNO 3 ) into 17.4mL of deionized water; when the silver nitrate was completely dissolved, weigh 60mg of sodium citrate, and dissolve it in an aqueous solution of silver nitrate under magnetic stirring; weigh 19mg of sodium borohydride and dissolve it in 10mL of deionized water , to obtain a 10mL concentration of 5×10 -2 mol / L sodium borohydride aqueous solution; under the environment of magnetic stirring, according to the ratio of the amount of reducing agent and metal ion substance being 1.3:1, add 2.6mL5×10 to the silver nitrate aqueous solution at one time -2 mol / L sodium borohydride aqueous solution, and then continue to react for 30min to obtain 20mL silver content of 5×10 -3 mol / L Ag nanoparticle sol.
[0045] 2. Preparation of fluorescent materials doped with Ag nanoparticles
[0046] 1) Fully mix 6.0 mL of distilled water and 30 mL of absolute ethanol at a ratio of 1:5 by volume, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com