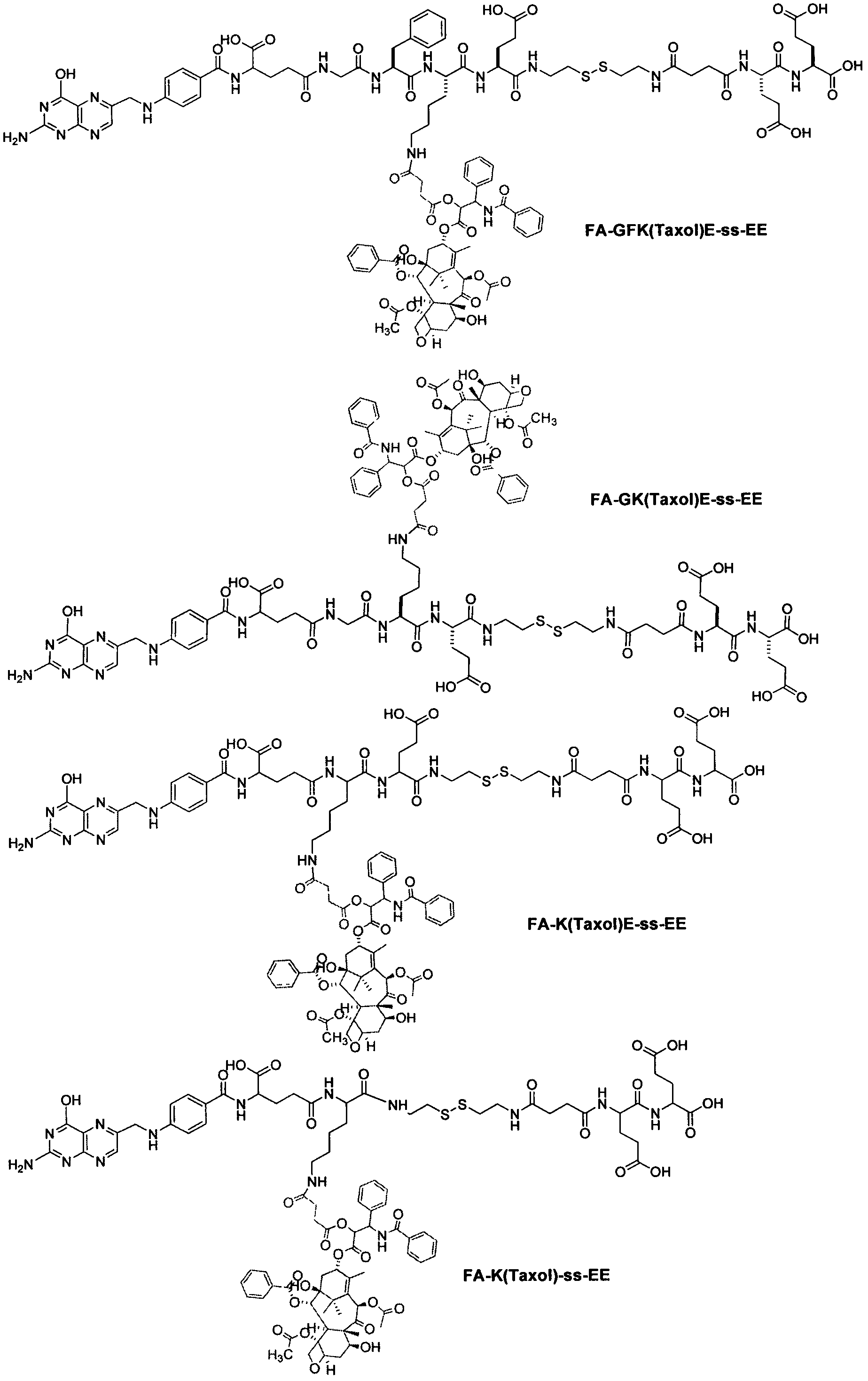
Preparation method of targeting folic acid-hydrophobic anticancer drug hydrogel
An anti-cancer drug, folic acid technology, applied in the preparation method of peptides, pharmaceutical formulations, anti-tumor drugs, etc., to achieve the effect of slow release of paclitaxel and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
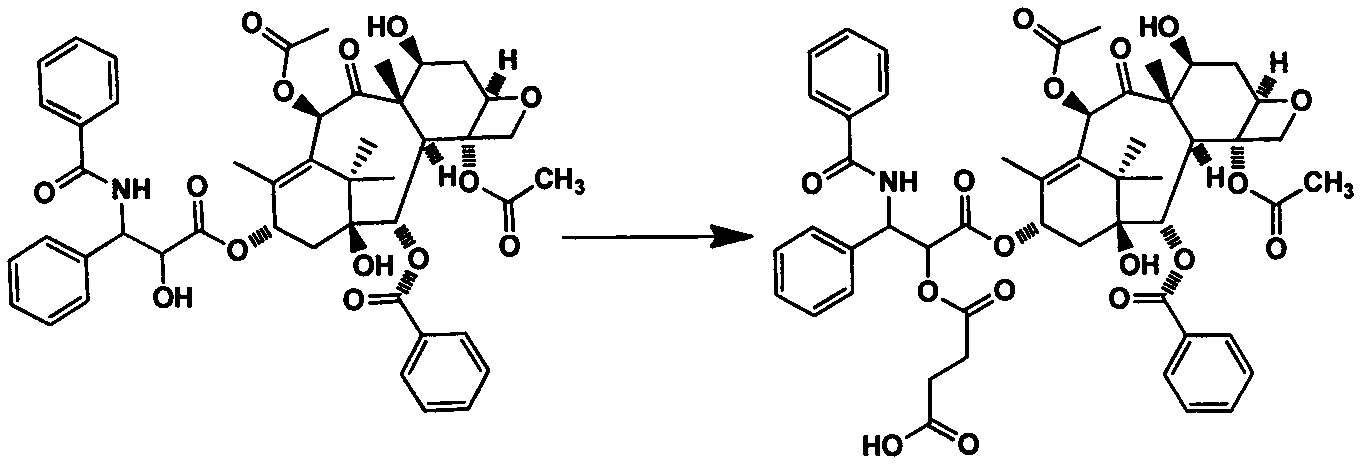
Embodiment 1
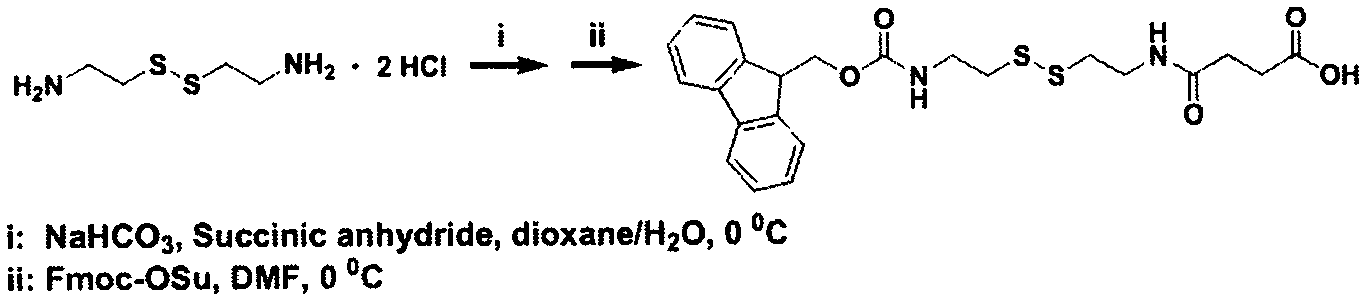
[0021] (1) For the synthesis of Fmoc-ss, see the attached instructions for the synthesis steps figure 1
[0022] The first step: the synthesis of ss
[0023] Take by weighing 10mmol (2.252g) Cystamine dihydrochloride and 30mmol (2.52g) sodium bicarbonate and dissolve it in the mixed solution of 90ml water and 35ml 1,4-dioxane to dissolve it, then weigh 10mmol (1.007g)1 , 4-succinic anhydride and dissolved in 3ml N,N-dimethylformamide, added to the above mixed solution, reacted overnight.
[0024] Step 2: Synthesis of Fmoc-ss
[0025] Weigh 10mmol (3.377g) fluorenyl methaneoxycarbonyl succinimide and dissolve it in 50ml acetone solution, slowly add the above reaction solution dropwise with a constant pressure funnel, react at room temperature for 4h, then filter to remove the white precipitate, and use a rotary evaporator Concentrate the filtrate by means of 1M hydrochloric acid to adjust the pH of the filtrate to acidity, separate out a white precipitate, let it stand for a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com