Medicinal composition containing trandolapril and preparation process thereof
A technology for trandolapril and composition, which is applied to the field of stable trandolapril capsules and their preparation, can solve the problems of not being able to fully meet the requirements of stable blood drug concentration, incomplete dissolution, etc., and achieves changes in instability and content reduction. The effect of falling, simple and practical process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
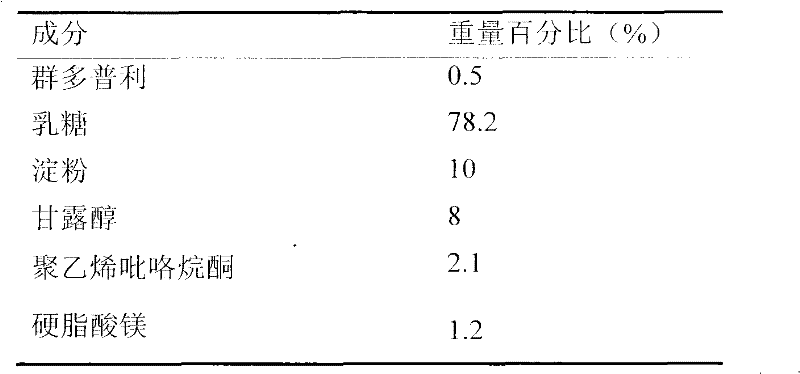
[0026]
[0027] Capsule preparation process: (1) Pass the main ingredient and magnesium stearate through 80-mesh sieves respectively, weigh them according to the proportion, add equal amounts and mix them evenly; (2) pass through 80-mesh sieves for lactose, starch and mannitol respectively Sieve, weigh according to the proportion, mix evenly, prepare 5% polyvinylpyrrolidone as a binder, wet granulate, granulate with a 16-mesh sieve, dry, and granulate with a 24-mesh. The two parts (1) and (2) are added in equal amounts, mixed evenly, and filled into capsules.
Embodiment 2
[0029]
[0030] Capsule preparation process: (1) pass the main ingredient, magnesium stearate, and talcum powder through an 80-mesh sieve, weigh according to the proportion, and mix uniformly in equal amounts; (2) lactose, microcrystalline cellulose, respectively Pass through an 80-mesh sieve, weigh according to the proportion, mix evenly, prepare 10% starch slurry as a binder, wet granulate, granulate with a 16-mesh sieve, dry, and granulate with a 24-mesh sieve. The two parts (1) and (2) are added in equal amounts, mixed evenly, and filled into capsules.
Embodiment 3
[0032]
[0033] Capsule preparation process: (1) pass the main drug and micropowder silica gel through 80-mesh sieve respectively, weigh according to the proportion, and mix evenly in equal amounts; (2) lactose, mannitol, low-substituted hydroxypropyl cellulose, Pass through 80-mesh sieve respectively, weigh according to the proportion, mix evenly, prepare 2% hydroxypropyl cellulose as a binder, wet granulate, granulate with 16-mesh sieve, dry, and granulate with 24-mesh. The two parts (1) and (2) are added in equal amounts, mixed evenly, and filled into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com