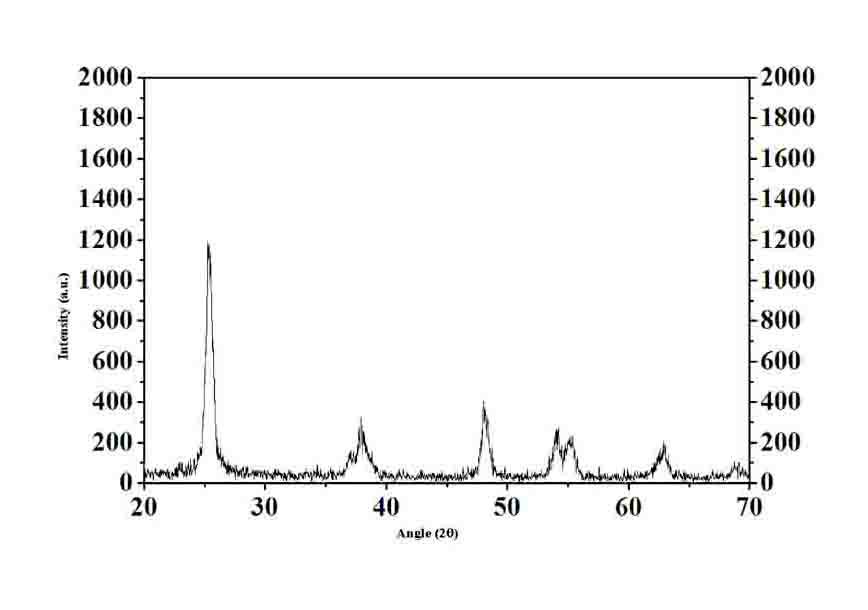
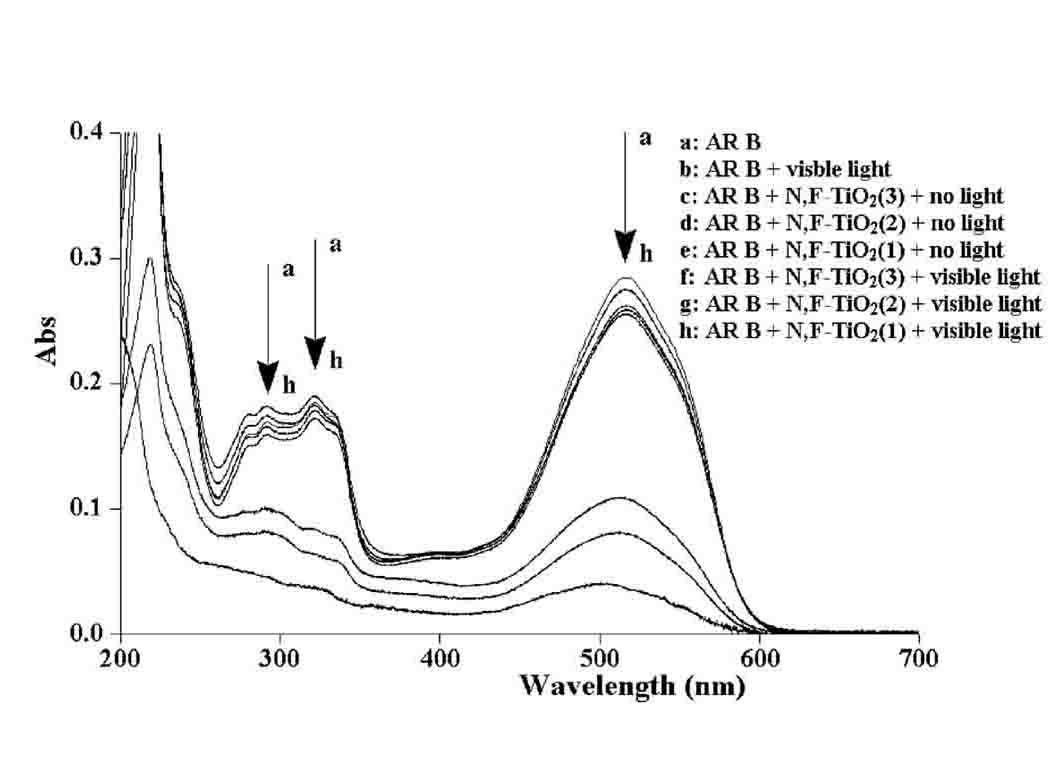
Nitrogen/fluorine-doped titanium dioxide photocatalyst and application thereof in degrading organic pollutants under visible light
A titanium dioxide, photocatalyst technology, applied in physical/chemical process catalysts, water pollutants, chemical/physical processes, etc., can solve the problems of low solar energy utilization, low quantum yield, etc., to promote the separation of photogenerated carriers, Good photocatalytic performance and the effect of reducing the recombination rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Nitrogen and fluorine doped titanium dioxide photocatalyst
[0025] (1) Preparation method
[0026] (1) Under rapid stirring, slowly drop 10 mL (0.03 mol) of butyl titanate into the mixed solution of 30 mL ethanol and 4.0 mL glacial acetic acid, stir for 30 min, and then add 5 ml dropwise with a concentration of 0.12 mol / L of hydrofluoric acid solution, stir to form a transparent mixed solution A; mix 5 ml of 0.12 mol / L ammonia with 10 ml of ethanol, adjust the pH to 2 with 1.0 mol / L nitric acid, and prepare solution B. The solution B is slowly dropped into the solution A to obtain a uniform transparent sol. Aging in the air for 24 hours to obtain a solid gel, drying at 80 ℃ for 12 hours, grinding into powder, and then placed in a muffle furnace 500 ℃ calcination 40 minutes, the molar percentage of N and Ti is 2%, F Nitrogen and fluorine doped titanium dioxide photocatalyst powder with a molar percentage of 2% to Ti. This catalyst is labeled N,F-TiO 2 (1).
...
Embodiment 2
[0043] Example 2 Nitrogen and fluorine doped titanium dioxide photocatalyst
[0044] Preparation method: Under rapid stirring, slowly drop 10 mL (0.03 mol) butyl titanate into the mixed solution of 30 mL ethanol and 4.0 mL glacial acetic acid, and stir for 30 min; then add 5 ml dropwise with a concentration of 0.06 mol / L hydrofluoric acid solution, stir to form a transparent mixed solution A; mix 5 ml of 0.06 mol / L ammonia water with 10 ml of ethanol, adjust the pH to 2 with 1 mol / L nitric acid, and prepare solution B. The solution B is slowly dropped into the solution A to obtain a uniform transparent sol. Aging in the air for 24 h to obtain a solid gel, drying at 80 ℃ for 12 h, grinding into powder, and then placing it in a muffle furnace at 500 ℃ for 40 min calcination, the molar percentages of N, F and Ti are 1%. The nitrogen and fluorine doped titanium dioxide photocatalyst.
[0045] Similarly, change the concentration of hydrofluoric acid solution and ammonia water to 0.1...
Embodiment 3
[0050] Example 3 Nitrogen and fluorine doped titanium dioxide photocatalyst
[0051] Preparation method: The method is the same as that of Example 1, but the difference is: calcination at 300°C, 400°C, 500°C, 600°C, and 700°C for 40 min.
[0052] Degradation experiment: adjust the concentration of Acid Red B to 10.0 mg / L and pH to 5.6; add nitrogen and fluorine doped titanium dioxide photocatalyst 1.5 g / L; visible light power is 128 W, and the irradiation time is 1.0 h. The degradation rate is shown in Table 4.
[0053] Table 4 The effect of different roasting temperatures on the degradation of Acid Red B
[0054] Roasting temperature (℃)300400500600700 Removal rate%10060.633.330.712.5
[0055] It can be seen from Table 4 that when the calcination temperature is 300°C, the removal rate of Acid Red B reaches 100%. This is because at lower temperatures, TiO 2 The crystallization has not been completed, the sample contains more amorphous TiO 2 Therefore, the catalyst has a certain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com