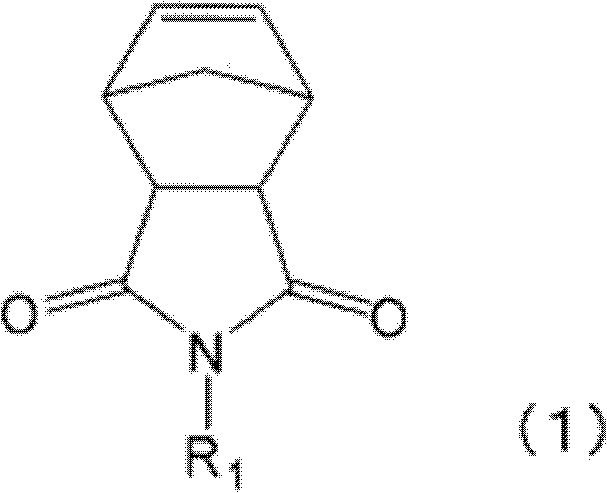
Polymer, hydrogen additive, resin composition, resin film, and electronic component
A resin composition and polymer technology, applied in the field of polymers, can solve problems such as use restrictions, and achieve the effects of high transparency, high adhesion, and low leakage current characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0156] The preparation method of the resin composition of this invention is not specifically limited, What is necessary is just to mix each component which comprises the resin composition of this invention by a well-known method.
[0157] The method of mixing is not particularly limited, but it is preferable to mix a solution or a dispersion obtained by dissolving or dispersing each component constituting the resin composition in a solvent. Thus, the resin composition of the present invention can be obtained in the form of a solution or a dispersion.
[0158] What is necessary is just to follow a conventional method about the method of dissolving or dispersing each component which comprises the resin composition of this invention in a solvent. Specifically, a method of stirring using a stirrer and a magnetic stirrer, a method of using a high-speed homogenizer, a disperser, a planetary mixer, a twin-shaft mixer, a ball mill, a three-roll mill, and the like are exemplified. In ...
Embodiment 1
[0207] 100 parts of N-(1-methylpentyl)-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxyimide, 1 , 2 parts of 5-hexadiene, (1,3-two imidazoline-2-indene)(tricyclohexylphosphine)benzylidene ruthenium chloride (synthesized by the method described in Org. Lett., volume 1, page 953, 1999) 0.02 part and diethylene glycol 400 parts of methyl ethyl ether were reacted at 80° C. for 4 hours while stirring to obtain a polymerization reaction liquid of polymer A1. The obtained polymer A1 had a polymerization conversion rate of 99.6%, a weight average molecular weight of 5,660, a number average molecular weight of 3,510, and a molecular weight distribution of 1.49.
[0208] Polymer A1 was evaluated for solubility in methyl isobutyl ketone (MIBK). Table 1 shows the evaluation results. Polymer A1 is soluble in MIBK at 25°C.
Embodiment 2
[0210] The polymerization reaction liquid of polymer A1 obtained in Example 1 was poured into an autoclave, stirred at 150° C. and a hydrogen pressure of 4 MPa for 5 hours, and hydrogenated to obtain hydride A2. The obtained hydride A2 had a weight average molecular weight of 7,010, a number average molecular weight of 4,590, a molecular weight distribution of 1.53, and a hydrogenation rate of 99.8%.
[0211] Hydride A2 was evaluated for methyl isobutyl ketone (MIBK) solubility. Table 1 shows the evaluation results. Hydride A2 is soluble in MIBK at 25°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com