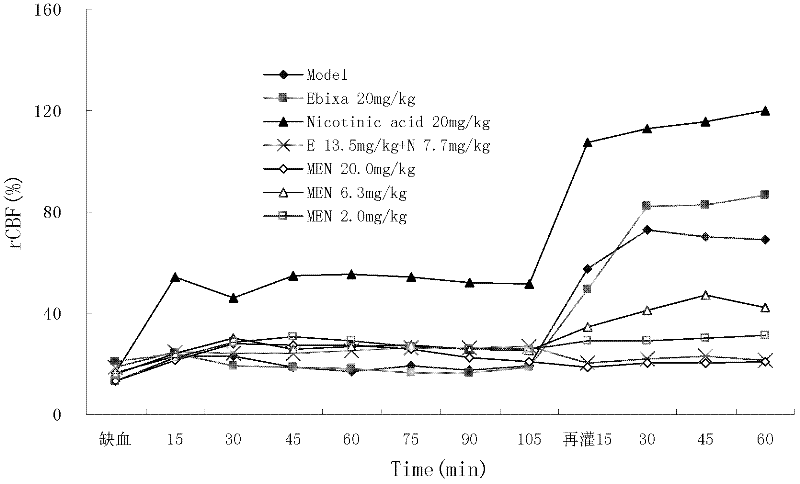
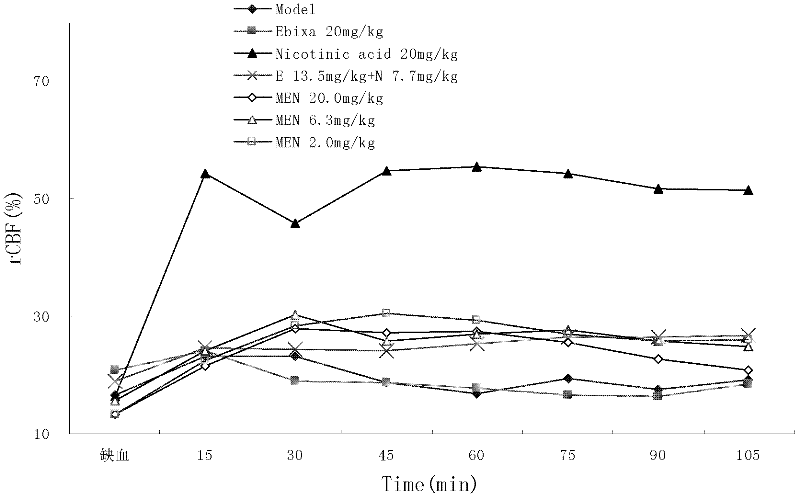
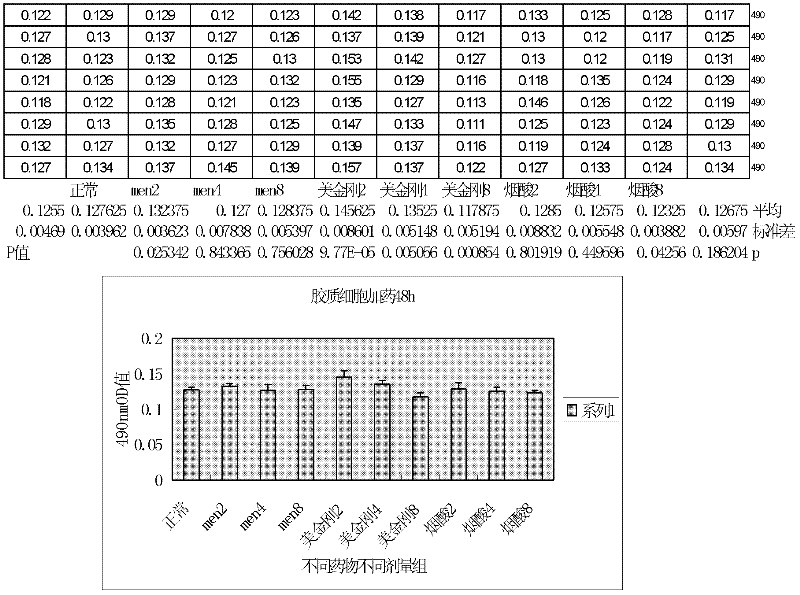
N-(3- pyridine formex oxygen radical)-3, 5- dimethyl-1- amantadine or pharmaceutically-acceptable salt for treating vascular dementia
A picolinyloxy, vascular dementia technology, applied to N-(3-picolinyloxy)-3,5-dimethyl-1-adamantanamine or a pharmaceutically acceptable salt thereof for the treatment of vascular dementia In the field of dementia, it can solve the problems of slow drug absorption and excretion, accumulation of poisoning and side effects, lack of ability to analyze and judge diseases, and achieve the effect of increasing distribution concentration and action time, social value and clinical value, and reducing peripheral adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Preparation of N-(3-pyridyloxy)-3,5-dimethyl-1-adamantanamine by DCC / DMAP method (active ester method)
[0215] 2.46g (0.02mol) of nicotinic acid and 4.32g (0.02mol) of memantine hydrochloride were dissolved in dry 120mL DMF at room temperature, 0.30g (0.002mol) of DMAP was added dropwise after adding 2.30mL of anhydrous TEA, and stirred evenly. Add 6.19g (0.03mol) DCC solution in 50mL DMF dropwise at 0-5°C, stir overnight at room temperature, filter, concentrate under reduced pressure, add an appropriate amount of ethyl acetate, filter, then water, 0.1N HCl, saturated NaHCO 3 , saturated brine for 3 times, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, flash column chromatography (silica gel H, washing solution: petroleum ether-acetone), to obtain N-(3-pyridineformyloxy)-3,5 - Dimethyl-1-adamantanamine, colorless viscous substance, 3.58 g, yield 63%.
[0216] MS (EI), m / z: 284 (M + ), 269 (M + -CH 3 ), 178 (C 12 h 20 NO + ), 106(C ...
Embodiment 2
[0222] Preparation of N-(3-pyridyloxy)-3,5-dimethyl-1-adamantanamine by CDI / DMAP method (active ester method)
[0223] Dissolve 0.62g (5mmol) niacin in dry 25mL DMF solution, quickly add 0.81g (0.002mol) CDI and 0.08g (0.5mmol) DMAP, and stir the reaction at 0-5°C for 1h. Add dropwise a mixed solution of 1.08g (5mmol) of memantine hydrochloride in 2.30mL of anhydrous TEA and 25mL of DMF solution, stir overnight at room temperature, concentrate under reduced pressure, add an appropriate amount of ethyl acetate, filter, and then water, 0.1N HCl, saturated NaHCO 3 , saturated brine for 3 times, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, flash column chromatography (silica gel H, washing solution: petroleum ether-acetone), to obtain N-(3-pyridineformyloxy)-3,5 - Dimethyl-1-adamantanamine, colorless viscous substance, 0.99 g, yield 70%.
Embodiment 3
[0225] Preparation of N-(3-pyridineformyloxy)-3,5-dimethyl-1-adamantanamine by mixed acid anhydride method
[0226] Dissolve 0.32g (2.60mmol) niacin in an appropriate amount of anhydrous CH under ice-salt bath 2 Cl 2 1mL (7.18mmol) TEA and 0.4mL (3.13mmol) anhydrous benzenesulfonyl chloride were slowly added dropwise in sequence, stirred for 1h, and 0.40g (1.86mmol) of memantine hydrochloride and TEA’s CH 2 Cl 2 The mixed solution was stirred for 3 h, concentrated under reduced pressure, added an appropriate amount of ethyl acetate, filtered, followed by water, 0.1N HCl, saturated NaHCO 3 , saturated brine for 3 times, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain a red oil, which was separated and purified by column chromatography (silica gel H, eluent: petroleum ether-acetone mixed solvent) to obtain N-(3-pyridinemethyl Acyloxy)-3,5-dimethyl-1-adamantanamine, colorless viscous substance, 0.41 g, yield 77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com