Garlic enteric-coated tablets and production method thereof
A production method and technology of enteric-coated tablets, which are applied in the field of garlic enteric-coated tablets and its production, can solve problems such as unguaranteed products, lower yields, and products that do not meet quality standards, and achieve the effects of qualified appearance and qualified disintegration time limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
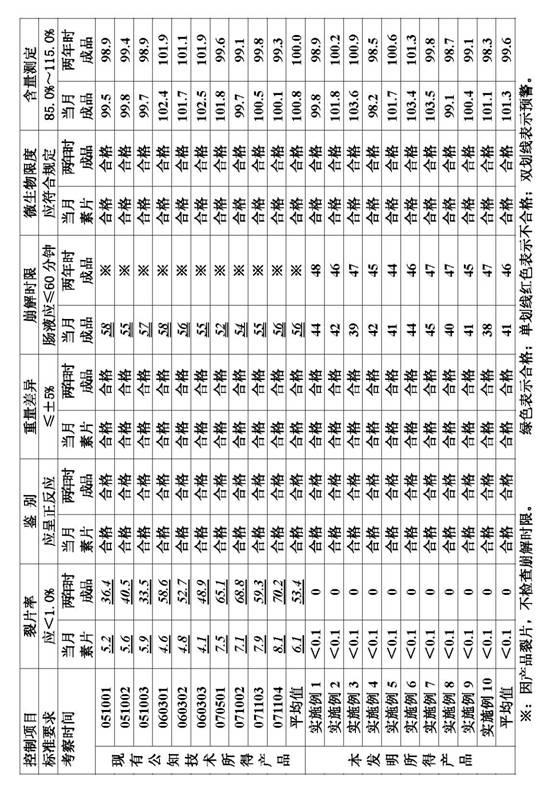
[0012] Example 1, the garlic enteric-coated tablets are obtained in parts by weight of the following raw materials: 65 parts by weight to 75 parts by weight of freeze-dried garlic powder, 10 parts by weight to 15 parts by weight of microcrystalline cellulose, and 2.2 parts by weight to pregelatinized starch. 3.3 parts by weight, 3.0 to 5.0 parts by weight of sodium carboxymethyl starch, 1.8 to 2.2 parts by weight of povidone K30, 0.8 to 1.2 parts by weight of silicon dioxide as a pharmaceutical excipient, and 0.2 parts by weight of magnesium stearate 0.3 parts by weight, 7 parts by weight to 8 parts by weight of pharmaceutical film coating premixed auxiliary materials.
Embodiment 2
[0013] Example 2, the garlic enteric-coated tablets are obtained in parts by weight of the following raw materials: 65 parts by weight of freeze-dried garlic powder, 10 parts by weight of microcrystalline cellulose, 2.2 parts by weight of pregelatinized starch, and 3.0 parts by weight of sodium carboxymethyl starch , 1.8 parts by weight of povidone K30, 0.8 parts by weight of silicon dioxide as a medical auxiliary material, 0.2 parts by weight of magnesium stearate, and 7 parts by weight of a medical film-coating premixed auxiliary material.
Embodiment 3
[0014] Example 3, the garlic enteric-coated tablets are obtained in parts by weight of the following raw materials: 75 parts by weight of freeze-dried garlic powder, 15 parts by weight of microcrystalline cellulose, 3.3 parts by weight of pregelatinized starch, and 5.0 parts by weight of sodium carboxymethyl starch , 2.2 parts by weight of povidone K30, 1.2 parts by weight of silicon dioxide as a medical auxiliary material, 0.3 parts by weight of magnesium stearate, and 8 parts by weight of a medical film-coating premixed auxiliary material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com