Preparation method of micrometer-scale cubic ultrodispersed ferroferric oxide particles
A technology of ferroferric oxide and cubes, which is applied in the field of preparation of ultra-dispersed ferroferric oxide particles, which can solve the problems of difficult preparation of ferric oxide particles and small particles of ferroferric oxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] A kind of preparation method of the ultra-dispersed iron ferric oxide particle that is a micron-sized cube of the present invention comprises the following steps:
[0019] 101. Dissolve ferric salt and ferrous salt with a molar ratio of 2:1 in deionized water to form a reaction solution.
[0020] 102. Add hydrogen peroxide to the reaction solution obtained in step 101. After reacting for 30 minutes, drop an alkaline solution into the reaction solution to adjust the pH of the reaction solution to 9-10 to form a suspension.
[0021] In this step, the concentration of hydrogen peroxide in the hydrogen peroxide is 30%, that is, the mass fraction of hydrogen peroxide in the hydrogen peroxide is 30%. The volume percentage of hydrogen peroxide and the reaction liquid is 0.6%-4%.
[0022] 103. Put the suspension obtained in step 102 in a stainless steel reaction kettle with a tetrafluoroethylene bottom pad, and put the stainless steel reaction kettle in an oven at 120-180°C. A...
Embodiment 1
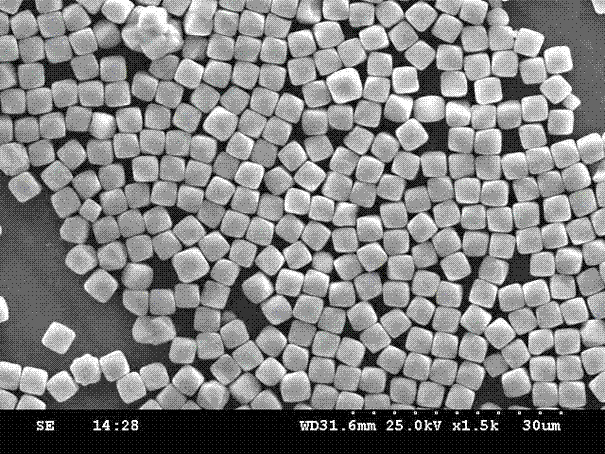
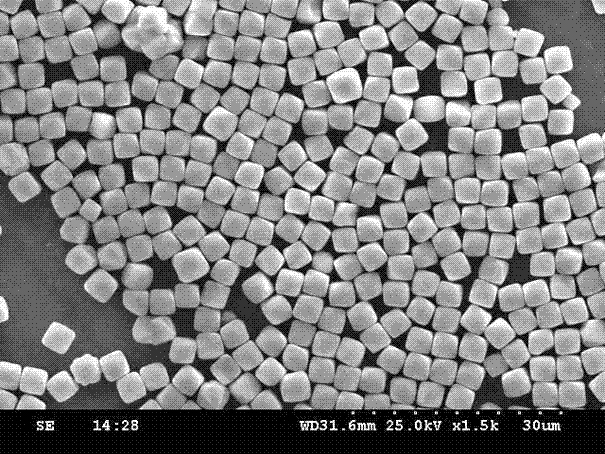
[0031] Dissolve 2.7 grams of ferric chloride and 1 gram of ferrous chloride in 100 mL of deionized water, then add 1 mL of hydrogen peroxide, react for 30 minutes, add 1 mL of ammonia water dropwise, and place the reaction solution on a 50 mL tetrafluoroethylene pad In a stainless steel reaction kettle, put the reaction kettle at 120°C for 12 hours, and then cool to room temperature to obtain cubic ferric oxide particles; wash the ferric oxide particles with deionized water three times to remove the residues on the surface of the particles The ferric ions were vacuum dried for 2 hours, placed in a muffle furnace at 300°C for 12 hours and then cooled to room temperature to obtain ferric iron tetroxide particles. The finally obtained iron ferric oxide particles were characterized by a scanning electron microscope with a magnification of 1500 times, as shown in figure 1 As shown, the particle size of the ferroferric oxide particles is 3 μm, the gap between the particles is large...
Embodiment 2
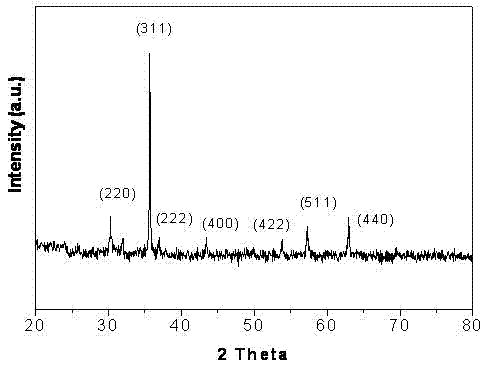
[0033] Dissolve 5.4 grams of ferric chloride and 2 grams of ferrous chloride in 100 mL of deionized water, add 1 mL of hydrogen peroxide, react for 30 minutes, drop 1 mL of 1mol / L NaOH solution, and place the reaction solution in 50 mL of tetrafluoroethylene Put the reaction kettle in a stainless steel reaction kettle with ethylene bottom pad, put the reaction kettle in an oven at 160°C and react for 12 hours, then cool to room temperature to obtain cubic ferric oxide particles, wash the ferric oxide particles three times with deionized water , remove iron ions remaining on the surface of ferric oxide particles, vacuum dry for 2 hours, place in a muffle furnace at 500°C for calcination for 6 hours, and obtain ferric oxide particles. Identical to the test method adopted in Example 1, the finally obtained ferric oxide particles are characterized by a scanning electron microscope and an X-ray diffractometer. The ferric oxide particles are cubic, and meanwhile, the particle diamete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com