Flavonoid compound and application thereof in preparation of pharmaceutical preparation
A flavonoid compound and a technology for preparing drugs are applied in the direction of drug combinations, antipyretics, anti-inflammatory agents, etc., and can solve the problems of poor water solubility of baicalin, which is not conducive to drug absorption, utilization, and preparation of pharmaceutical preparations, etc. Good sex and strong pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
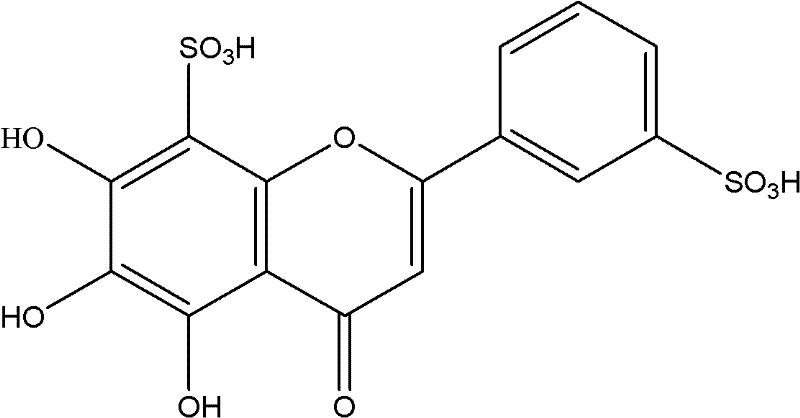
[0203] One kilogram of baicalin was dissolved in 20 liters of fuming sulfuric acid, stirred at 80°C for 8 hours, neutralized with saturated sodium hydroxide, concentrated under reduced pressure, extracted with 20 liters of ethanol, and obtained a yellow powder product ( 0.9 kg).
[0204] The chemical structure identification data of the yellow powder product:
[0205]
[0206] 5,6,7-Trihydroxy, 8,3′-disulfonic acid flavone
[0207] Molecular weight: MW=430, molecular formula is C 15 h 10 o 11 S 2
[0208] Mass Spectrum: ESI-MS (negative ion mode): m / z 451[M-2H+Na]-, 429[M-H]-; Secondary Mass Spectrum: 429[M-H]-, 349[M-SO3H]-, 319, 181 .
[0209] NMR data: 1H-NMR (400MHz) and 13C-NMR (100MHz) data (DMSO-d6)
[0210]
[0211]
[0212]
[0213] Hydrocarbon long-range correlations (HMBC correlations) of compounds of the present invention
[0214] It is identified by the above spectroscopic method that the yellow powder product is 5,6,7-trihydroxy, 8,3'-disulfo...
Embodiment 2
[0216] 5,6,7-Trihydroxy, 8,3′-disulfoflavone injection:
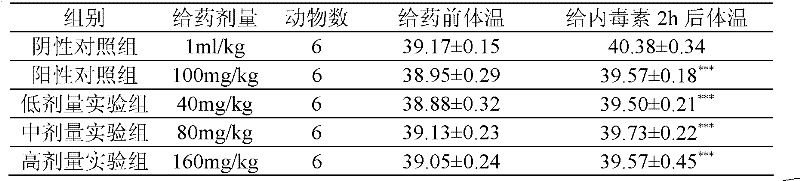
[0217] Take 5000mg of 5,6,7-trihydroxyl, 8,3′-disulfonic acid flavone, dissolve it in 500ml of water to make an aqueous solution, adjust the pH to 5.5-6.5, heat to dissolve, mix well, and divide into 100mg / 10ml / branch of injection, sterilized by steam circulation for 30 minutes.
[0218] Usage and dosage: intramuscular injection or intravenous injection, 100-1000mg per day.
Embodiment 3
[0220] 5,6,7-trihydroxy, 8,3'-disulfonic acid flavone oral solution:
[0221] Dissolve 50,000mg of 5,6,7-trihydroxyl,8,3′-disulfonic acid flavone in 2,000ml of water to make a 2.5% aqueous solution, adjust the pH to 5.5-6.5, heat to dissolve, mix well, and fill in 10ml In the medicine bottle, seal and sterilize.
[0222] Method of administration: take orally, take 250-1500 mg of 5,6,7-trihydroxyl, 8,3′-disulfonic acid flavone daily.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com