Preparation method of acyclic nucleoside antiviral drug phosphoric acid monoester compound
A technology for antiviral drugs and cyclic nucleosides, applied in the field of medicine, can solve problems such as being difficult to industrialize production, and achieve the effects of easy industrial production, low price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
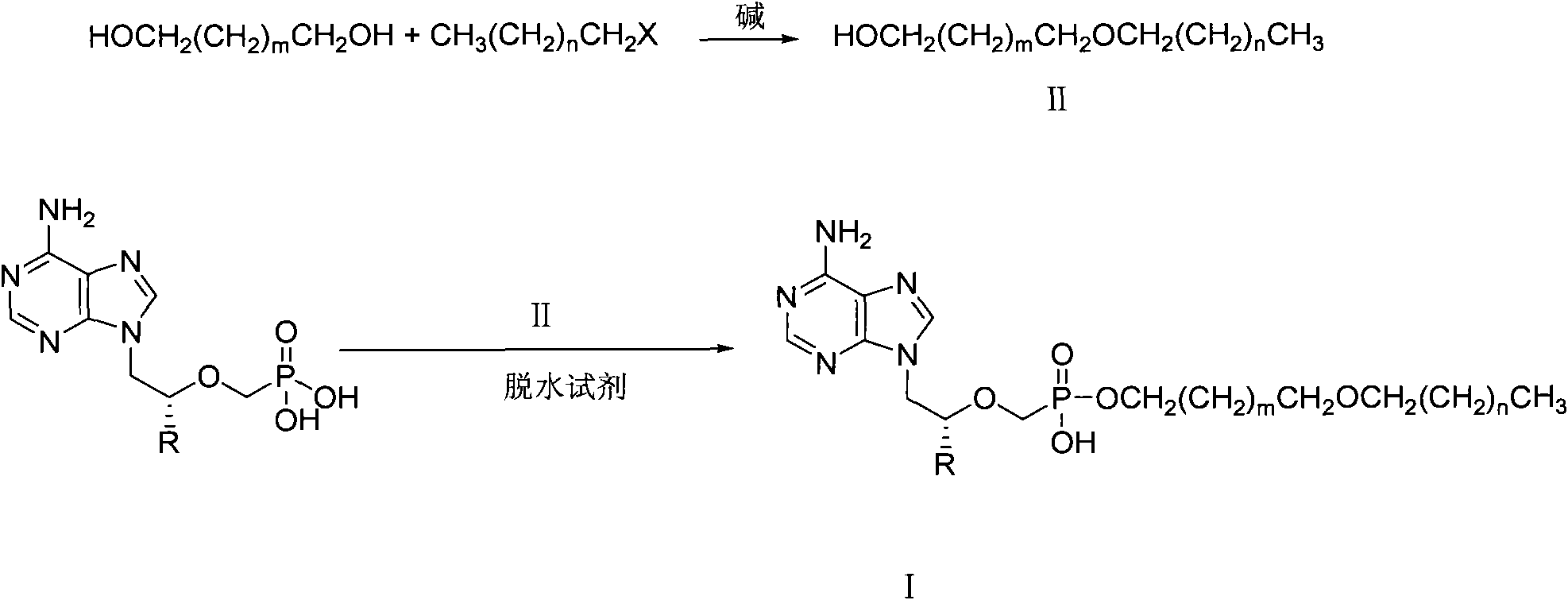
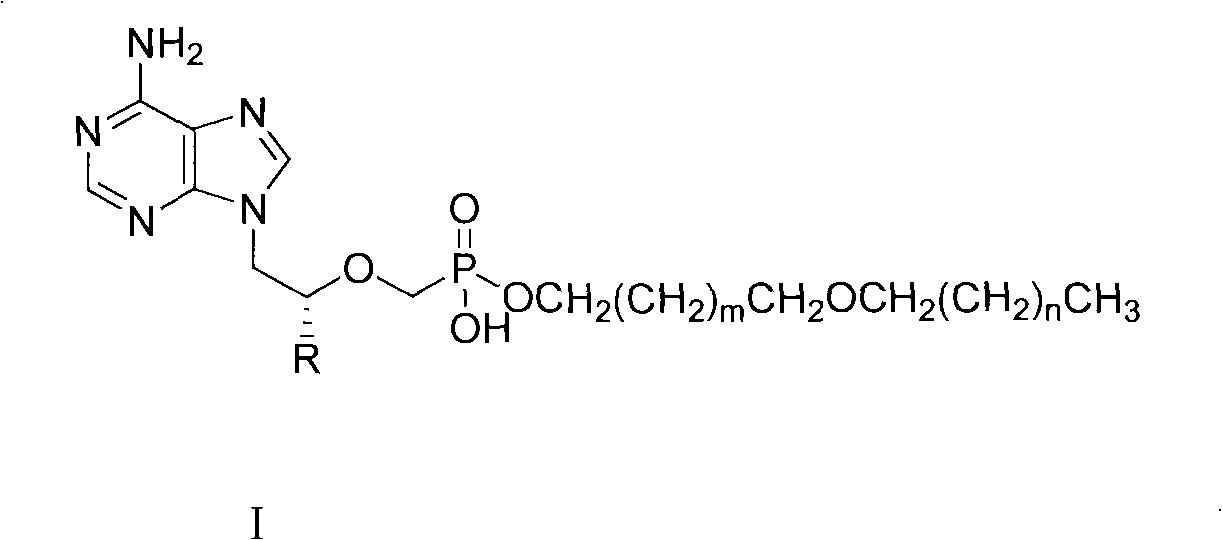
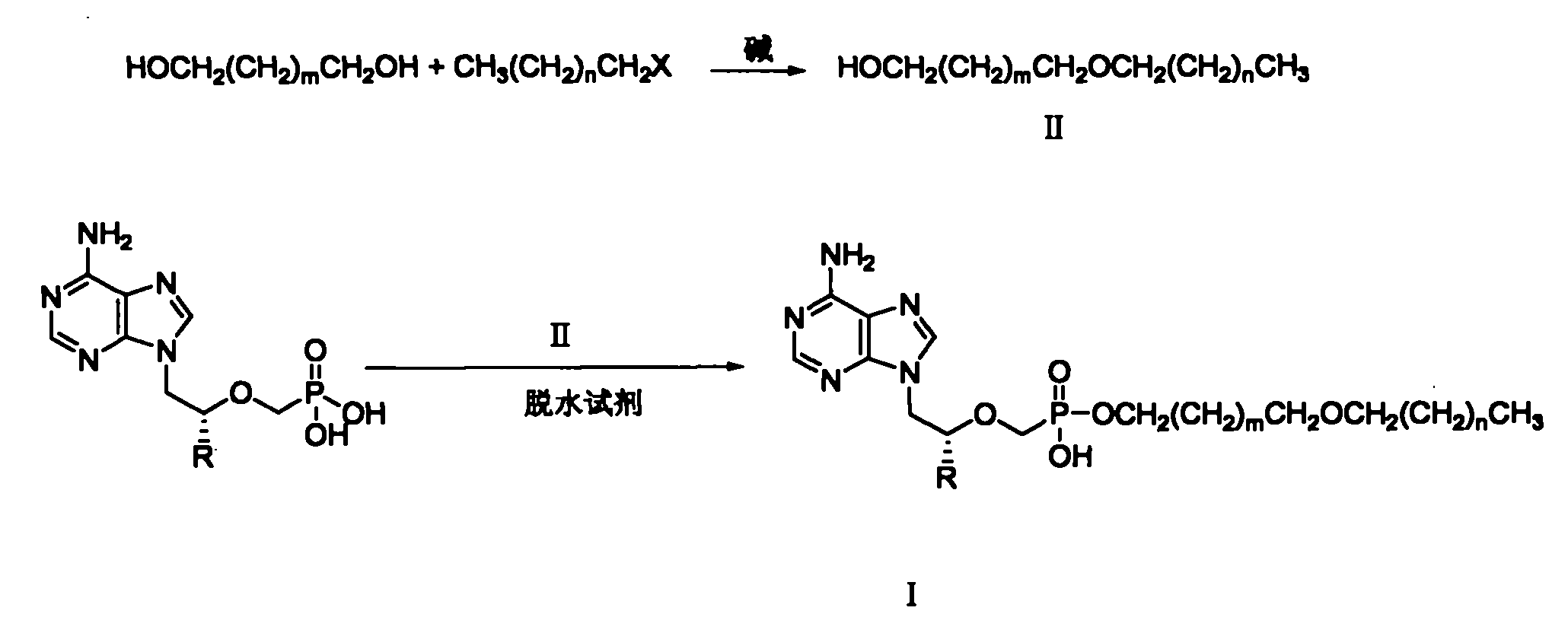
[0030] Example 1: Synthesis of 2-octadecyloxy-1-ethanol
[0031] Bromooctadecane (214.8g, 0.64mol) and ethylene glycol (120g, 1.93mol) were dissolved in 400ml DMSO and 400ml tetrahydrofuran (THF), KOH (144g, 2.56mol) was added, and mechanically stirred at room temperature for 16h. Pour 400 ml of deionized water into the reaction solution, and neutralize it with 18% HCl (about 250 ml). extracted with ethyl acetate, washed with saturated brine, and washed with anhydrous MgSO 4 dry. After filtration, the filtrate was spin-dried to obtain a pale yellow waxy solid. The compound was recrystallized from petroleum ether to obtain 90 g of the compound as a white solid (yield 43%). Mp: 49-51°C. 1 H-NMR (CDCl 3 )δ(ppm): 0.88(t, J=6.4Hz, 3H), 1.25(m, 30H), 1.58(m, 2H), 3.47(t, J=6.8Hz, 2H), 3.53(t, J= 4.8Hz, 2H), 3.72(t, J = 4.4Hz, 2H).
Embodiment 2
[0032] Embodiment 2: the synthesis of 3-hexadecyloxy-1-propanol
[0033] Using hexadecane bromide and 1,3-propanediol as raw materials, and using sodium hydroxide as a base, 3-hexadecyloxy-1-propanol was synthesized in a similar manner to Example 1. Yield 82%, mp: 37-40°C. 1 HNMR (CDCl 3 )δ (ppm): 0.880 (t, 3H), 1.256 (broad singlet, 24H), 1.427 (s, 1H), 1.566 (p, 2H), 1.818 (p, 4H), 3.409-3.795 (3 groups 3 heavy peak, 6H).
Embodiment 3
[0034] Embodiment 3: the synthesis of 3-tetradecyloxy-1-propanol
[0035] Using tetradecane bromide and 1,3-propanediol as raw materials and sodium carbonate as base, 3-tetradecyloxy-1-propanol was synthesized in a similar manner to Example 1. Yield 85%, mp: 32-34°C; 1 HNMR (CDCl 3 )δ (ppm): 0.873 (t, 3H), 1.309 (broad singlet, 22H), 1.558 (p, 2H), 1.823 (p, 2H), 2.503 (s, 1H), 3.401-3.783 (3 groups 3 heavy peak, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com