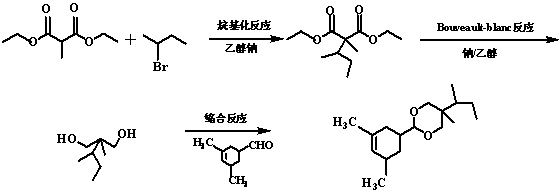
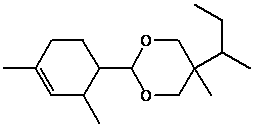
Method for synthesizing karanal
A technology of carrageenaldehyde and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of low yield and the like, and achieve the effects of wide sources, low cost and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] A complete synthesis method of caralanal is provided, and the specific synthesis route is divided into three steps:
[0013] (1) Synthesis of intermediate (I) 2-methyl-2-sec-butyl-diethyl malonate: Add 0.33g-0.66g sodium metal to 25mL-80mL absolute ethanol, and wait for the metal Add 2.11g-4.17g diethyl 2-methylmalonate after complete reaction of sodium, stir and reflux for 30min-50min, add 2.49g-4.95g 2-bromobutane, and reflux at 80-90℃ React for 4h-5h. After the reaction is over, cool the reaction mixture to room temperature, distill off the solvent ethanol, add 1-3 times the volume of the mixture to dilute with water, and then extract 3 times with diethyl ether that is 1-3 times the volume of the diluted liquid, and combine the extractions liquid, and the extract was washed twice with distilled water and saturated NaCl aqueous solution, dried to remove water, and filtered to remove impurity NaCl, distilled ether in the filtrate to obtain 2-methyl-2-sec-butyl-propaned...
Embodiment 1
[0017] (1), the synthesis of intermediate (I) 2-methyl-2-sec-butyl-diethyl malonate:
[0018] Weigh 0.33g of sodium metal and slowly put it into a 50mL three-necked bottle (room temperature) filled with 25mL of absolute absolute ethanol. After all the sodium has reacted, 2.11g of diethyl 2-methylmalonate was slowly added dropwise with a constant pressure dropping funnel, stirred and refluxed for 30min, and then 2.49g of 2-bromobutane was slowly added dropwise. , reflux reaction 4h, during the reaction, track the reaction process with GC and TLC;
[0019] After the reaction, the reaction mixture was cooled to room temperature, the solvent ethanol was distilled off, and an equal volume of the mixture was added to dilute with water, and then extracted three times with an equal volume of dilute liquid ether. After combining the extracts, they were washed with distilled water and saturated NaCl aqueous solution respectively. Extract the solution twice, dry and remove the moisture ...
Embodiment 2
[0026] (1), the synthesis of intermediate (I) 2-methyl-2-sec-butyl-diethyl malonate:
[0027] Weigh 0.50g of sodium metal and slowly put it into an 80mL three-necked bottle (room temperature) filled with 50mL of absolute absolute ethanol. After all the sodium has reacted, slowly drop 3.10g of 2-methyldiethylmalonate into it with a constant pressure dropping funnel, stir and reflux for 40min, then slowly add 3.83g of 2-bromobutane dropwise, at 85°C, reflux Reaction 4.5h, during the reaction, track the reaction process with GC and TLC;
[0028]After the reaction, the reaction mixture was cooled to room temperature, the solvent ethanol was evaporated, diluted with water twice the volume of the mixture, extracted three times with diethyl ether twice the volume of the diluted liquid, and the combined extracts were washed with distilled water and saturated NaCl aqueous solution respectively. 2 times, dry to remove water, filter to remove impurity NaCl, and distill off the ether in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com