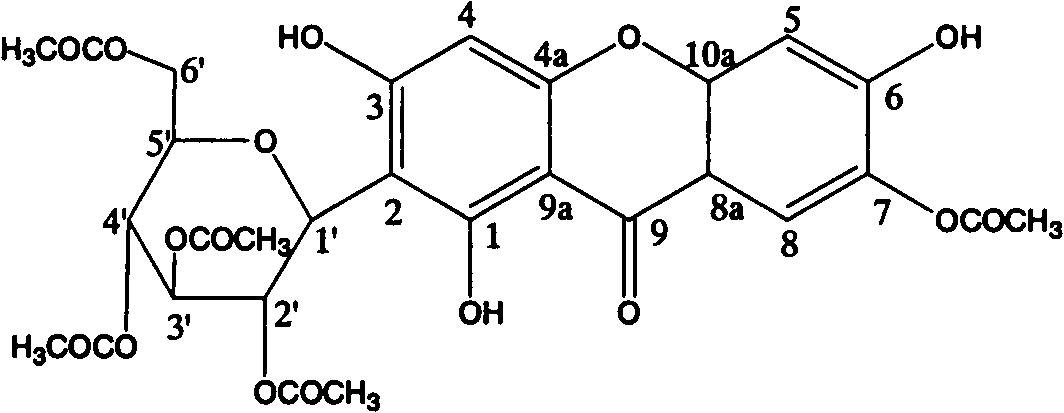
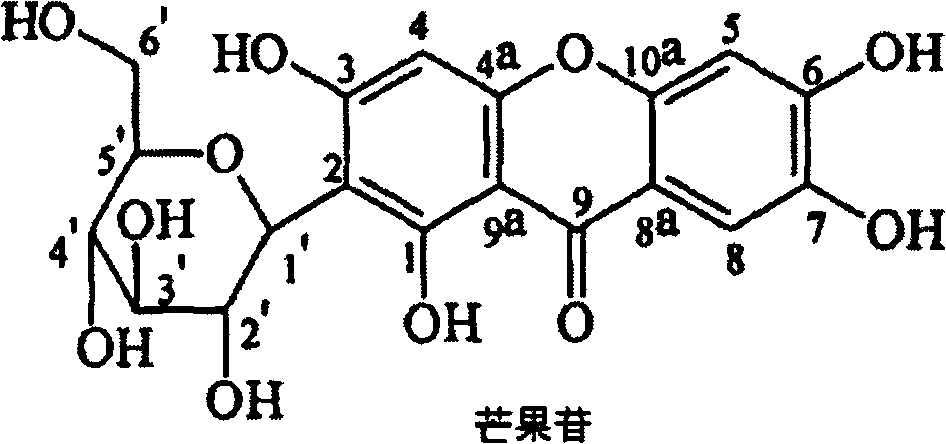
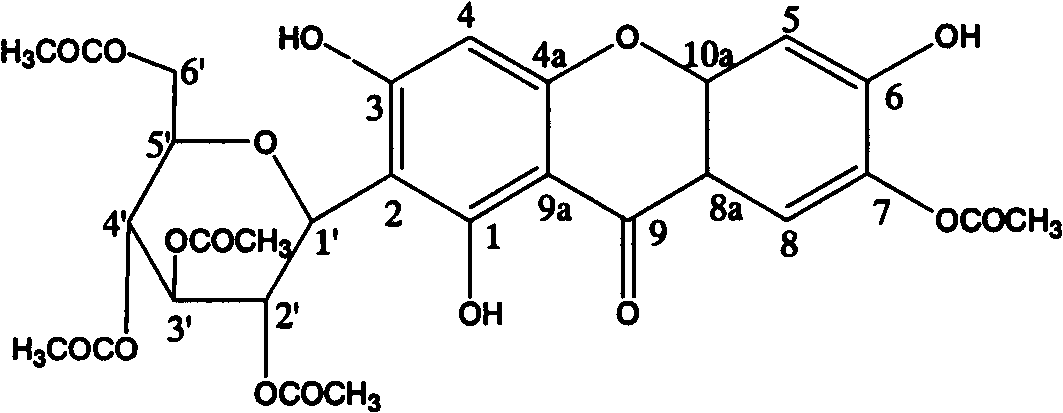
Mangiferin penta-esterified derivative
A technology of glycoside pentaethyl ester and derivatives, applied in the field of mangiferin pentaethyl esterification derivatives, can solve the problems of poor permeability of mangiferin, poor gastrointestinal permeability of mangiferin, and poor absorption effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Preparation of mangiferin pentaethyl esterified derivatives
[0014] Operate in a water bath, add 1.5ml of 98% H to 250ml of acetic anhydride dropwise under constant stirring 2 SO 4 , then add 10g of mangiferin after dripping; place the solution at 40°C to keep warm, react for 24 hours, stir from time to time to dissolve all the mangiferin; after the reaction is complete, pour the reaction solution directly into water under constant stirring ; The insoluble matter was collected by filtration to obtain the reaction product; the reaction product was mixed with silica gel as a sample, put on the top of the silica gel dry column, and was first eluted with chloroform, followed by elution with chloroform: ethyl acetate: acetone=20: 1: 1, and then Elute with chloroform: ethyl acetate: acetone = 7:2:1, collect fractions, evaporate to dryness to obtain crude crystals; recrystallize the crude crystals with methanol 2-3 times to obtain mangiferin pentaethyl esterified ...
Embodiment 2
[0015] Example 2: Preparation of mangiferin pentaethyl esterified derivatives
[0016] Operate in a water bath, add 4ml of 36% HCl dropwise to 250ml of acetic anhydride under constant stirring, and then add 10g of mangiferin after dropping; keep the solution at 50°C for 12 hours, stirring occasionally Dissolve the mangiferin completely; after the reaction is completed, pour the reaction solution directly into water under constant stirring; filter the insoluble matter to obtain the reaction product; mix the reaction product with silica gel, put it on the top of the silica gel dry column, and first use chloroform Elution, followed by elution with chloroform: ethyl acetate: acetone = 20: 1: 1, then elution with chloroform: ethyl acetate: acetone = 7: 2: 1, collected fractions, evaporated to dryness, to obtain crude crystals; The crude crystals are recrystallized 2-3 times with methanol to obtain mangiferin pentaethyl esterified derivatives.
Embodiment 3
[0017] Embodiment 3: anti-inflammatory pharmacological experiment and result
[0018] Mouse auricle swelling test 40 mice were randomly divided into 4 groups. Set up normal control group, aspirin group (0.045g / kg), large and small doses of mangiferin pentaethyl ester derivatives group (0.4g / kg, 0.2g / kg), intragastric administration once a day, normal control group The group was given an equal volume of distilled water for 7 consecutive days. 0.5 h after the last administration, 20 μl of xylene was dripped on the right ear of the mouse with a micropipette, and the mouse was killed by pulling the cervical spine after 15 min. Immediately use a hole punch with a caliber of 6 mm to punch holes along the same part of the left and right auricles of the mice, and then weigh them with an electronic balance of 1 / 10,000, and take the difference in weight (mg) between the two ears as the index of swelling degree , swelling degree=(right ear weight-left ear weight) / right ear weight, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com