Recombinant antigenic protein for detecting dengue virus antibody, kit and application thereof
A technology of recombinant antigenic protein and detection kit, applied in the field of genetic engineering, can solve problems such as difficulties in immunological diagnosis and differential diagnosis, difficulty in infection with dengue virus, and limited coverage, so as to facilitate quality control, ensure sensitivity and specificity, highly specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
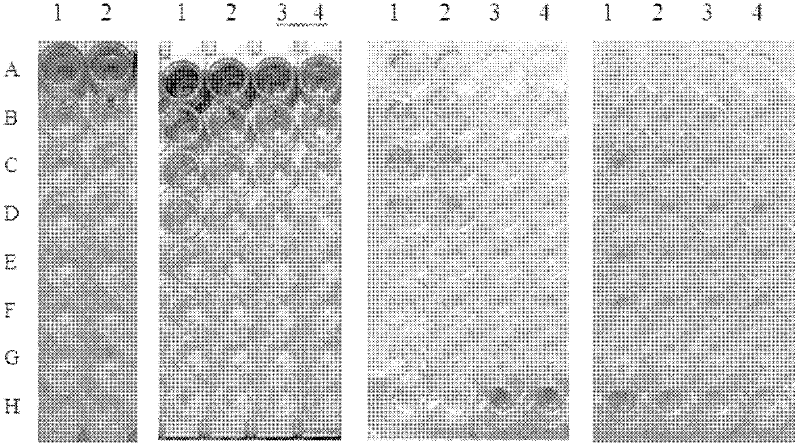
[0045] Embodiment 1, detect the preparation of the recombinant antigenic protein of dengue virus
[0046] Prepare the recombinant antigen protein for detecting dengue virus according to the following steps:
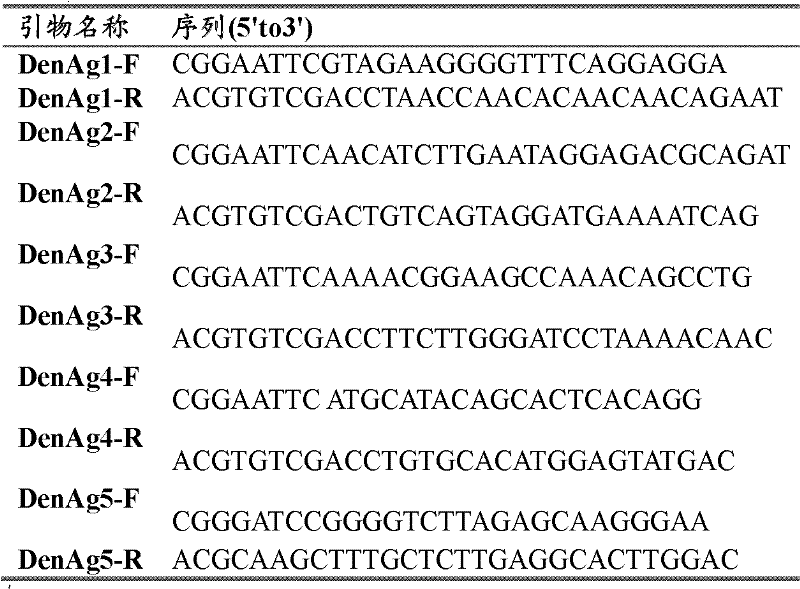
[0047] Step 1, select the coding gene presenting the highly specific peptide of the virus in the dengue virus protein as the target gene fragment, which includes the extended segment of the flexible arm of the recombinant antigen protein, and the flexible arm can eliminate the expression vector. The masking of the polypeptide epitope by the fusion protein enables the correct folding of the expressed recombinant antigen protein. Wherein, the target gene fragment includes the coding sequence of Den-Ag5, the coding sequence of Den-Ag1 and the coding sequence of Den-Ag2, and the coding gene sequence of Den-Ag1 has the nucleotide sequence shown in SEQ ID NO.1; The coding gene sequence of Den-Ag2 has the nucleotide sequence shown in SEQ ID NO.3; the coding gene sequence of Den...
Embodiment 2
[0075] Embodiment 2, the coating experiment of recombinant antigenic protein
[0076] In this example, it is necessary to optimize the coating conditions and concentration of the recombinant antigenic protein of the present invention; in order to achieve this purpose, it is also necessary to first explain the components of the ELISA reaction solution in the kit:
[0077] Enzyme conjugate working solution: horseradish peroxidase-labeled anti-human IgG polyclonal antibody;
[0078] Positive control: Dengue fever patient serum.
[0079] Negative control: dengue virus antibody negative human serum.
[0080] Sample diluent: 10mmol / L pH7.4 phosphate buffer containing 1% BSA, 0.05% Tween-20 and 1mg / L gentamicin;
[0081] Concentrated washing solution: 0.1mol / L pH7.4 phosphate buffer containing 1% fetal bovine serum, 0.5% Tween-20 and 20mg / L gentamicin;
[0082] Chromogenic solution A: 0.02% H2O2; dilute with 0.1M citric acid-0.2M disodium hydrogen phosphate, pH4.5~5.0.
[0083] C...
Embodiment 3
[0088] Embodiment 3, ELISA reaction condition optimization
[0089] The antigen coating conditions and ELISA reaction conditions were systematically optimized, and the best ELISA reaction conditions were finally determined as follows: recombinant antigen 100ul (concentration: 10ug / ml) coated overnight at 4°C, washed with PBST for 3 minutes, patted dry, 5% BSA 37 Block at ℃ for 2 hours, add 100ul of the serum to be tested into the reaction well after 1:100 times dilution, react at 37°C for 1 hour, wash with PBST 4 times, each time for 1 minute, pat dry, after the HRP-labeled secondary antibody is diluted 1:10000 times, Add 100ul to each well, react at 37°C for 40 minutes, wash with PBST 4 times, 1 minute each time, pat dry, add 100ul of TMB substrate solution to each well, react at 37°C for 15 minutes, add 50ul 2M H 2 SO 4 The reaction was terminated, and the OD value was measured with a microplate reader at a wavelength of 450 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com