Terlipressin acetate nasal cavity spray and preparation method thereof
A technology of terlipressin and nasal spray, which is applied in aerosol delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve problems such as inconvenient use, patient pain and high economic burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 The formulation of terlipressin acetate nasal spray is: 1.0 mg / ml terlipressin acetate, 0.1% w / v disodium edetate, 0.80% w / v sodium chloride, 0.30% w / v chlorobutanol, acetic acid to adjust pH to 5.5.
[0016] Its preparation process is as follows: Accurately weigh 100mg disodium edetate, 800mg sodium chloride, and 300mg chlorobutanol in a 1000ml beaker, add purified water to fully dissolve, then add 1000mg terlipressin acetate, and It is fully dissolved, and an appropriate amount of acetic acid is added to adjust the pH value to 5.5, and the purified water is constant to volume. Filter through a 0.22 μm microporous membrane, fill it into a sterilized spray bottle after passing the inspection, and add a bottle cap with a spray metering pump; label and pack to obtain terlipressin acetate nasal spray.
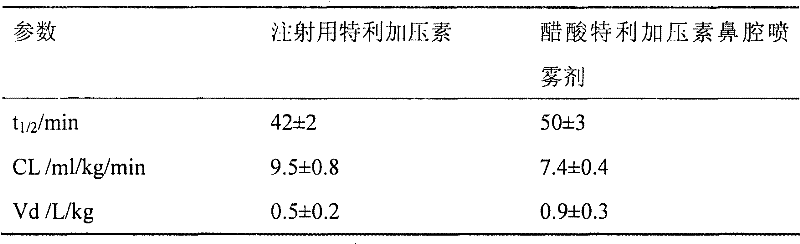
[0017] Ten sprays of nasal spray containing 1.0 mg / ml terlipressin acetate and ten injections of terlipressin for injection were prepared according to this examp...
Embodiment 2
[0025] Example 2 The formulation of terlipressin acetate nasal spray is: 1.0 mg / ml terlipressin acetate, 0.5% w / v sodium deoxycholate, 1.0% w / v glucose, 0.40% w / v Chlorobutanol, acetic acid regulation pH is 5.5, and its preparation process is as follows:
[0026] The preparation process is as follows: Accurately weigh 500mg sodium deoxycholate, 1000mg glucose, and 400mg chlorobutanol in a 1000ml beaker, add purified water to fully dissolve it, then add 1000mg terlipressin acetate to fully dissolve it, A proper amount of acetic acid was added to adjust the pH to 5.5, and the purified water was made to volume. Filter through a 0.22 μm microporous membrane, fill it into a sterilized spray bottle after passing the inspection, and add a bottle cap with a spray metering pump; label and pack to obtain terlipressin acetate nasal spray.
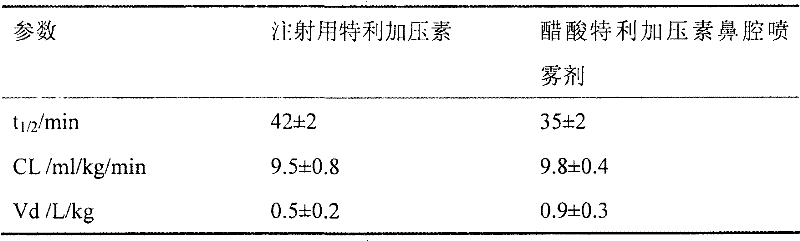
[0027] Ten sprays of nasal spray containing 1.0 mg / ml terlipressin acetate and ten injections of terlipressin for injection were prepared according ...
Embodiment 3
[0035] Example 3 The formulation of terlipressin acetate nasal spray is: 1.5 mg / ml terlipressin acetate, 0.5% w / v disodium edetate, 0.5% w / v chitosan, 1.0% W / v sodium chloride, 0.30% w / v chlorobutanol, acetic acid to adjust pH to 6.5, its preparation process is as follows:
[0036] The preparation process is as follows: Accurately weigh 500mg disodium EDTA, 500mg chitosan, 1000mg sodium chloride, and 300mg chlorobutanol in a 1000ml beaker, add purified water to fully dissolve, then add 1500mg tertiary acetate For vasopressin, fully dissolve it, add an appropriate amount of acetic acid to adjust the pH to 6.5, and dilute to volume with purified water. Filter through a 0.22 μm microporous membrane, fill it into a sterilized spray bottle after passing the inspection, and add a bottle cap with a spray metering pump; label and pack to obtain terlipressin acetate nasal spray.
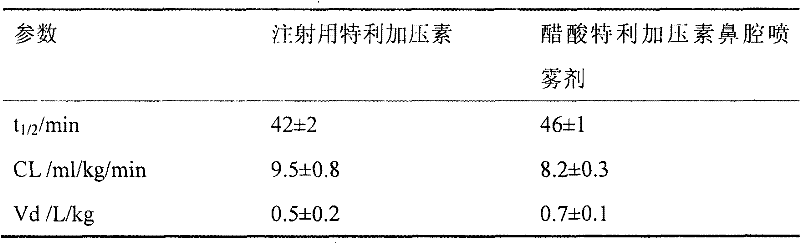
[0037] 10 sprays of 1.5 mg / ml terlipressin acetate nasal spray and 10 injections of terlipressin for inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com