Racemization method of S-lipoic acid and preparing method of R-lipoic acid
A technology of racemization and lipoic acid, applied in the direction of organic chemistry methods, chemical instruments and methods, organic racemization, etc., can solve the problems of relatively harsh production conditions, difficult to obtain pure products, high production costs, etc., and achieve good results Industrial applicability, reducing safety hazards, avoiding the effect of processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
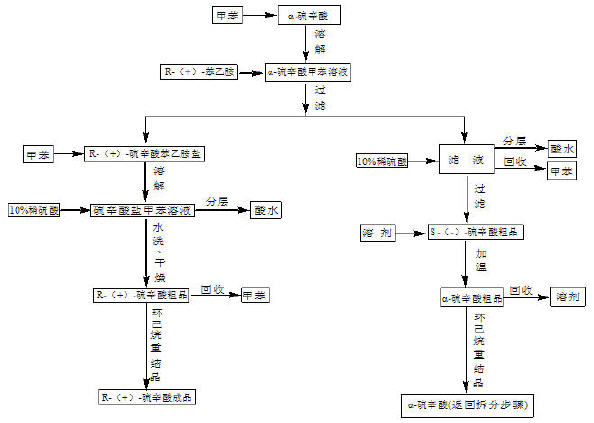
[0042] Weigh 100g of racemic lipoic acid, dissolve it in 500g of toluene at 60°C, slowly add 50g of R-(+)-phenethylamine dropwise, and finish dropping in about 1.5 hours. Cool slowly to 0-10°C, filter, and wash the filter cake with toluene to obtain about 110 g of R-(+)-lipoic acid phenethylamine salt. Dissolve the above product in 500g toluene at 40°C, add 10% dilute sulfuric acid solution dropwise until the pH value is 2-3, let it stand for half an hour, and then remove the acid water. The toluene layer was washed with water until neutral, dried over anhydrous sodium sulfate, and filtered. The toluene was recovered by distillation under reduced pressure, and the obtained crude product was recrystallized with 600g of cyclohexane to obtain 45g of R-(+)-lipoic acid product, with a yield of 45%.
[0043] The filtered mother liquor was acidified with 10% dilute sulfuric acid to a pH value of 2-3, washed, concentrated toluene, crystallized and filtered to obtain about 50g of S-(-...
Embodiment 2
[0046] About 50g of S-(-)-lipoic acid was prepared in Example 1, dissolved in 100ml of toluene, heated to reflux at a reflux temperature of 110°C for 22 hours, and the solvent was concentrated. Recrystallized with cyclohexane to obtain 45g, =-0.072°.
[0047]
Embodiment 3
[0049]According to Example 1, about 50 g of S-(-)-lipoic acid was prepared, dissolved in 200 ml of DMF, heated to reflux at a reflux temperature of 153° C. to 156° C., refluxed for 22 hours, and concentrated the solvent. Recrystallized with cyclohexane to obtain 43g, =-0.043°.
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com