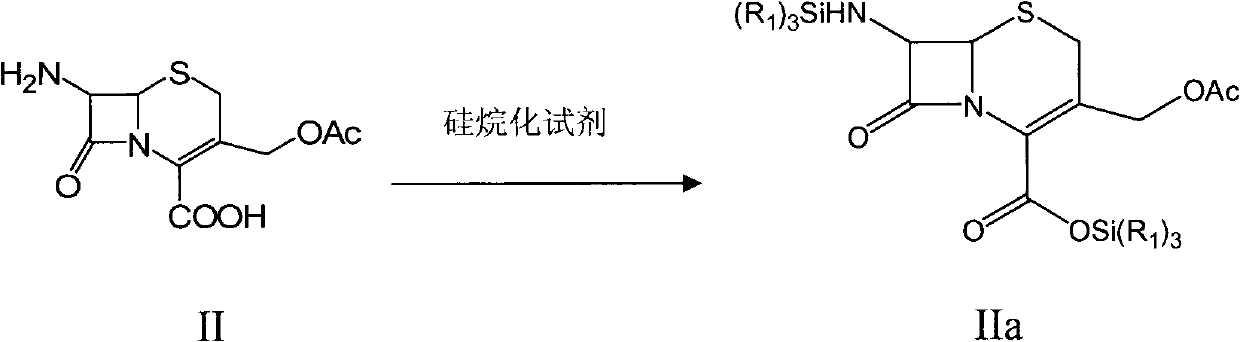
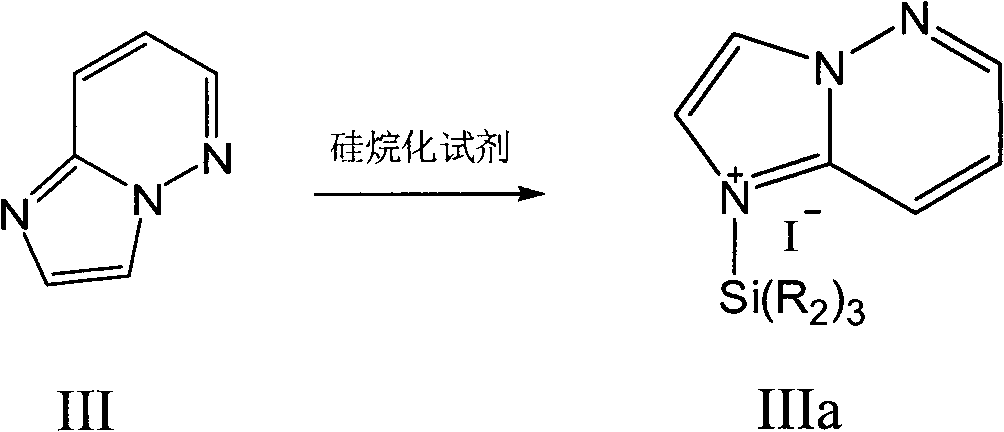
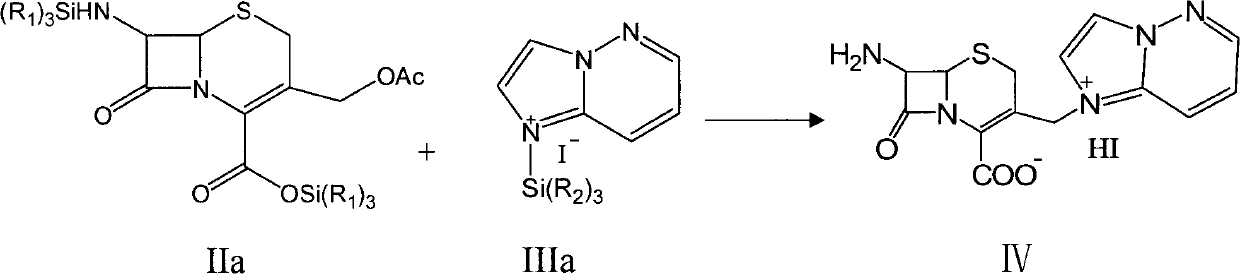
Preparation method of cefozopran hydrochloride
A technology of ceftozolam hydrochloride and cefozopran hydrochloride, which is applied in the field of preparation of cefazolam hydrochloride, can solve the problems of reducing moisture content, reducing solvent residue, difficult moisture content, etc., to reduce the difficulty of reaction purification and facilitate industrial production , The effect of improving the utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of cefozopran hydrochloride
[0041] (1) Under the protection of nitrogen, add 10L of dichloromethane and 1kg of 7-ACA into a 20L reaction kettle, under stirring at 25°C, add 0.89kg of hexamethyldisilazane, add 25g of methanesulfonic acid dropwise, and then heat to 55 ℃ reaction for 3 hours, and the reaction solution was set aside;
[0042] (2) Under the protection of nitrogen, add 8L of dichloromethane and 0.88kg of imidazo[1,2-b]pyridazine in a 30L reaction kettle, and add 1.47kg of iodotrimethylsilane under stirring at 20°C, and heat up to React at 30°C for 5 hours, and the reaction solution is set aside;
[0043] (3) Add the reaction solution in step (1) to the reaction solution in step (2), and keep it at about 30°C for 3 hours. After the reaction was completed, the reaction solution was cooled to about 0°C, and 1.5L of methanol was added dropwise, then stirred for 1 hour, filtered, and the filter cake was washed twice with pre-coole...
Embodiment 2
[0046] Embodiment 2: the preparation of cefozopran hydrochloride
[0047] (1) Under the protection of nitrogen, add 10L of dichloromethane and 1Kg of 7-ACA into a 20L reaction kettle, under stirring at 25°C, add 1.12kg of N,O-bistrimethylsilylacetamide (BSA), and react at 25°C for 1 hour, the reaction solution is standby;
[0048] (2) Under nitrogen protection, add 8L of dichloromethane and 1.05kg of imidazo[1,2-b]pyridazine into a 30L reaction kettle, add 1.47Kg of iodotrimethylsilane under stirring at room temperature, and heat up to 35°C React for 4 hours, and the reaction solution is ready for use;
[0049] (3) Add the reaction solution in step (1) to the reaction solution in step (2), and keep it at about 35°C for 3 hours. After the reaction was completed, the reaction liquid was cooled to about 0°C, and 1.5L of methanol was added dropwise, stirred for 1 hour, filtered, and the filter cake was washed twice with pre-cooled 1L of methanol, drained, and vacuum-dried to obt...
Embodiment 3
[0052] Embodiment 3: the preparation of cefozopran hydrochloride
[0053] (1) Add 10L of dichloromethane and 1kg of 7-ACA into a 20L reaction kettle under nitrogen protection, add 1.47kg of iodotrimethylsilane under stirring at 25°C, then react at 25°C for 2 hours, and the reaction solution is set aside;
[0054] (2) Add 8L of dichloromethane and 1.31kg of imidazo[1,2-b]pyridazine into a 30L reaction kettle under nitrogen protection, add 1.47kg of iodotrimethylsilane under stirring at room temperature, and heat up to 40°C for reaction 3 hours, the reaction solution is ready for use;
[0055] (3) Add the reaction solution in step (1) to the reaction solution in step (2), and keep it at about 40°C for 3 hours. After the reaction was completed, the reaction liquid bath was cooled to about 0°C, and 1.5L of methanol was added dropwise, stirred for 1 hour, filtered, and the filter cake was washed twice with pre-cooled 1L of methanol, drained, and vacuum-dried to obtain 1-[[ (6R,7R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com