Method for preparing oligofuran dioctyl phthalate ester by directly esterifying and polymerizing
A technology of polyfurandicarboxylate and furandicarboxylic acid, which is applied in the fields of high molecular polymer materials and chemical engineering, can solve the problems of polymerization technology and rare products, and achieve the effect of high-efficiency purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
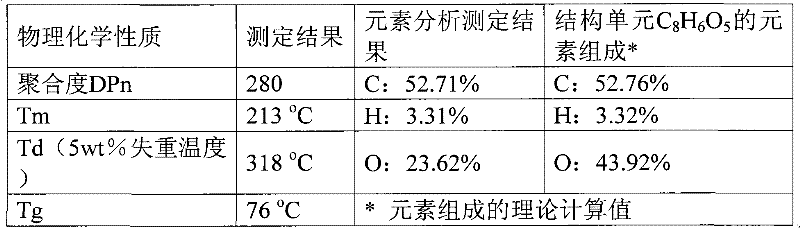
[0021] Example 1: 10 mmol of 2,5-furandicarboxylic acid, 20 mmol of 1,2-ethylene glycol, and 0.1 mmol of PbO were added to a 50 mL round bottom flask. Under the protection of nitrogen, it was heated to 140°C; it was reacted at this temperature for 4h. Then the excess ethylene glycol and other impurities were distilled off under reduced pressure. The temperature was raised to 230°C, the vacuum was adjusted to 5mmHg, and the reaction was continued for 3h. Finally, 2 g of o-xylene was added, and the reaction was continued for 1 h under the above conditions. Cool to room temperature, add 10g of trifluoroacetic acid to dissolve, then drop the trifluoroacetic acid polymer solution into 60ml of methanol for precipitation, centrifuge, decant and remove the supernatant, the obtained polymer polyethylene furandicarboxylate The diester was a milky white solid and was dried under vacuum at 50°C for 12h. Calculated according to the feeding amount of the monomer 2,5-furandicarboxylic aci...
Embodiment 2
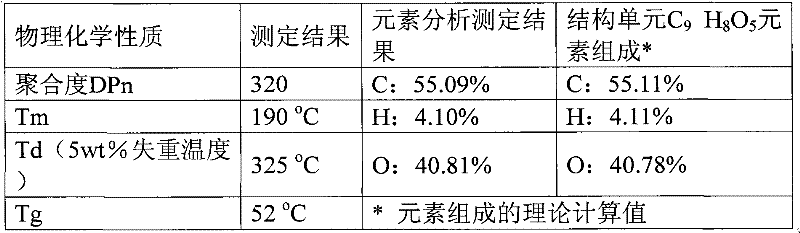
[0024] Embodiment 2: 10mmol 2,5-furandicarboxylic acid, 11mmol 1,3-propanediol, 0.03mmol ZnCl 2 Add it into a 50mL round bottom flask, heat to 160°C under nitrogen protection, react for 5h, then distill off excess propylene glycol under reduced pressure, raise the temperature to 210°C, adjust the vacuum to 3mmHg, continue the reaction for 2.5h, and finally add 4g biphenyl, continue to react under the above conditions for 1h. Cool to room temperature, add 5g of trifluoroacetic acid to dissolve, then drop its trifluoroacetic acid solution into 60ml of methanol for precipitation and separation, centrifuge, decant out the supernatant to obtain the polymer polytrimethylene furandicarboxylate as a milky white solid, Dry in vacuum at 50°C for 12h. Calculated according to the feeding amount of monomer 2,5-furandioic acid, the molar yield of the polymer is 96%. NMR spectrogram measurement results and attribution analysis are: 1 H NMR (TFA-d 1 , ppm): 2.05 (s, 2H, -O-CH 2 -CH 2 -)...
Embodiment 3
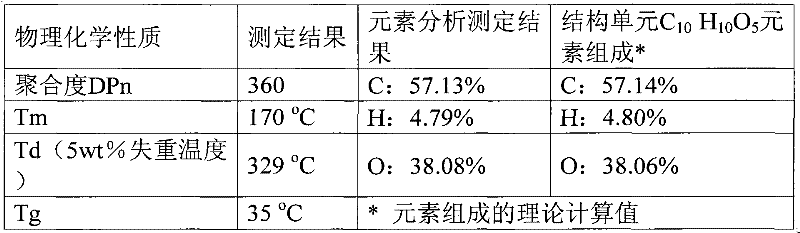
[0027] Embodiment 3: with 10mmol 2,5-furandicarboxylic acid, 30mmol 1,4-butanediol, 0.08mmolCa(OAc) 2 Added to a 50mL round bottom flask. Heated to 180°C under nitrogen protection and reacted for 6h. Excess butanediol was then distilled off under reduced pressure. The temperature was raised to 200°C, the vacuum degree was adjusted to 4mmHg, and the reaction was continued for 3.5h. Finally, 5 g of chlorobenzene was added, and the reaction was continued for 1 h under the above conditions. Cool to room temperature, add 15g of trifluoroacetic acid to dissolve, then drop its trifluoroacetic acid solution into 60ml of methanol for precipitation, centrifuge, decant out the supernatant to obtain the polymer polybutylene furandicarboxylate as a milky white solid. Dry in vacuum at 50°C for 12h. Calculated according to the feeding amount of monomer 2,5-furandicarboxylic acid, the molar yield of the polymer is 92%. NMR spectrogram measurement results and attribution analysis are: 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com