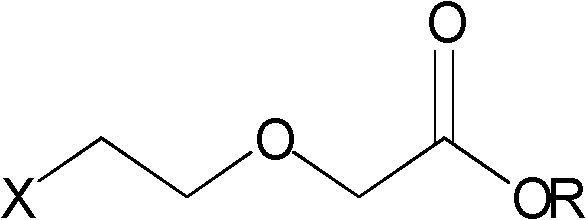
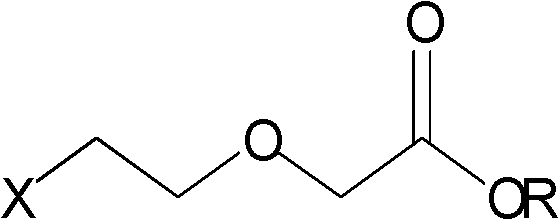
Preparation of haloethoxyacetic acid and esters thereof
A technology of haloethoxy and acetic acid, applied in the field of preparation of haloethoxy acetic acid and its ester, can solve problems such as explosiveness, unfavorable production, explosion, etc., and achieve the effects of low production cost, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 200g (1.6mol) 2-(2-chloroethoxy)ethanol, 5g (0.032mol) 2,2,6,6-tetramethyl-1-piperidinyloxy radical, 80ml water into the three-necked flask, Add 100g (0.64mol) sodium dihydrogen phosphate dihydrate under stirring, cool down to 20-25°C, slowly add the solution prepared by dissolving 218g (2.4mol) sodium chlorite in 500ml water into the above mixture, and control The temperature does not exceed 45°C. After the dropwise addition, the temperature is controlled at 20-35°C, kept for 2 hours, extracted with 400ml×3 dichloromethane, the extract is dried over anhydrous sodium sulfate, filtered, and the filtrate is concentrated under reduced pressure until the flow is cut off to obtain 2 -(2-Chloroethoxy)acetic acid 200.5g, yield 90.1%, GC purity 98.7%.
[0038] Pour the 2-(2-chloroethoxy)acetic acid obtained from the above concentration into a three-necked flask, add 1500ml of absolute ethanol, add 6.9ml (0.128mol) of concentrated sulfuric acid dropwise while stirring, and h...
Embodiment 2
[0040] Add 500g (4mol) 2-(2-chloroethoxy)ethanol, 6.2g (0.04mol) 2,2,6,6-tetramethyl-1-piperidinyloxy radical, 400ml water in the three-necked flask, Add 248g (1.59mol) sodium dihydrogen phosphate dihydrate under stirring, lower the temperature to 20°C-25°C, and add dropwise the solution prepared by dissolving 491g (5.42mol) sodium chlorite in 800ml water into the above mixture, and control The temperature does not exceed 45°C. After the dropwise addition, the temperature is controlled at 35-40°C, kept for 1 hour, and concentrated under reduced pressure until the flow is cut off. The concentrated mixture was extracted with 1150 ml of n-butanol, and suction filtered to obtain a n-butanol solution of 2-(2-chloroethoxy)acetic acid. Then add 1350ml of n-butanol, dropwise add 10.9ml (0.2mol) of concentrated sulfuric acid, reflux at 115-125°C for 9 hours, cool to 30-40°C, neutralize with saturated sodium bicarbonate solution, concentrate under reduced pressure to remove the solvent,...
Embodiment 3
[0042]Add 50g (0.4mol) 2-(2-chloroethoxy)ethanol, 0.62g (0.004mol) 2,2,6,6-tetramethyl-1-piperidinyloxy radical, 16ml water in the three-necked flask , add 24.8g (0.159mol) sodium dihydrogen phosphate dihydrate under stirring, cool down to 20 ℃ ~ 25 ℃, dissolve 49.1g (0.542mol) sodium chlorite in 80ml water and add dropwise to the above mixture Inside, control the temperature not to exceed 45°C, after the dropwise addition, control the temperature to 25-35°C and keep warm for 3 hours, concentrate under reduced pressure, add 110ml of isopropanol, and filter with suction to obtain isopropanol of 2-(2-chloroethoxy)acetic acid solution. Then add 130ml of isopropanol, add 1.5ml (0.02mol) of thionyl chloride dropwise, heat up to 65-70°C for reflux reaction for 8 hours, cool to 30-40°C, neutralize with saturated sodium bicarbonate solution, and depressurize Concentrate to remove the solvent, extract with 750ml×3 ethyl acetate, wash the ethyl acetate extract with 150ml×3 water, separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com