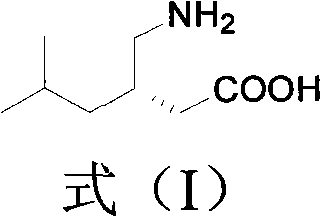
Preparation method of intermediate compound of pregabalin
A technology of pregabalin and compounds, applied in the field of preparation of intermediate compounds, can solve the problems of high cost, dangerous operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
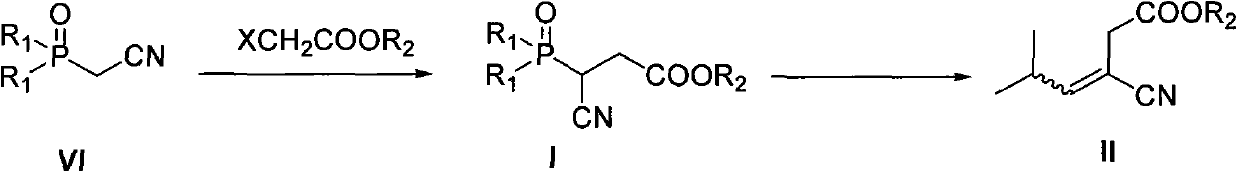
[0055] Embodiment 1: Synthesis of intermediate I (3-cyano-3-(diethoxyphosphoryl) ethyl propionate)
[0056] Sodium hydride (13.5 g, 60%, 339 mmol) and toluene (500 mL) were added into a three-necked reaction flask equipped with a thermometer and a stirrer under nitrogen protection. To the obtained suspension was added dropwise a solution of diethyl cyanomethylphosphate (30.0 g, 169 mmol) dissolved in 40 mL of toluene at -5-0°C. After the addition, keep within this temperature range, stir and react for 1 hour to obtain a thick mixture. Ethyl chloroacetate (41.5 g, 339 mmol) was dissolved in 40 mL of toluene solution and added dropwise to the above system, and the reaction mixture gradually became clear. After the dropwise addition, the temperature was raised to 10-15°C, followed by TLC or GC detection, and the reaction was complete in about 3 hours. The reaction was quenched with 0.1M aqueous HCl, the layers were separated and the organic layer was collected. The aqueous pha...
Embodiment 2
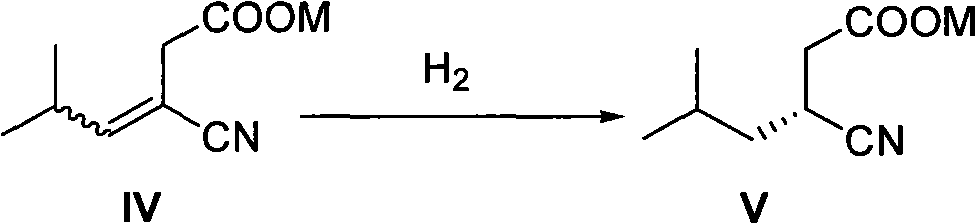
[0058] Example 2: Synthesis of intermediate II (3-cyano-5-methyl-3-ene-hexanoic acid ethyl ester)
[0059] Sodium hydride (1.73 g, 60%, 43.2 mmol) and toluene (40 mL) were added into a three-necked reaction flask equipped with a thermometer and a stirrer under nitrogen protection. Stir. Intermediate I (9.3 g, 35.4 mmol) dissolved in 30 ml toluene was added dropwise to the obtained suspension at -5-0°C. After the addition was complete, the temperature was maintained and stirring was continued for 1 hour. To the reaction mixture was added a solution of isobutyraldehyde (2.8 g, 38.9 mmol) dissolved in 30 ml of toluene. After the addition, the stirring reaction was continued for 2 hours, and detected by TLC or GC. When the phosphate ester reaction was complete, it was quenched by the careful addition of water 0.1M aqueous HCl. The organic layer was separated, and the aqueous phase was extracted with toluene. The organic phases were combined and dried over anhydrous sodium sul...
Embodiment 3
[0061] Example 3: One-pot synthesis of intermediate II (3-cyano-5-methyl 3-ene-hexanoic acid ethyl ester)
[0062] In a three-necked reaction flask equipped with a thermometer and a stirrer, under nitrogen protection, sodium hydride (27.1 g, 60%, 678 mmol) and toluene (1200 mL) were added. To the obtained suspension was added dropwise a solution of diethyl cyanomethylphosphate (100.0 g, 565 mmol) dissolved in 250 ml of toluene at -5-0°C. After the addition, keep within this temperature range, stir and react for 1 hour to obtain a thick mixture. Ethyl chloroacetate (83.1 g, 678 mmol) was slowly added dropwise to the above system, and the reaction mixture gradually became clear. After the dropwise addition, the temperature was raised to 10-15°C. After 1 hour, the temperature was lowered to 0°C. At 0-5°C, sodium hydride (22.6g, 60%, 565mmol) was added in batches, and the temperature was raised to 10-15°C after the addition was completed. The reaction was continued at ℃, followe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com