Ginsenoside Re extraction and separation method
A technology for extracting ginsenosides and solvents, which is applied in the field of extraction and separation of ginsenoside Re, can solve the problems of not being suitable for industrial production, and achieve the effects of environmental protection, low production cost, and short cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
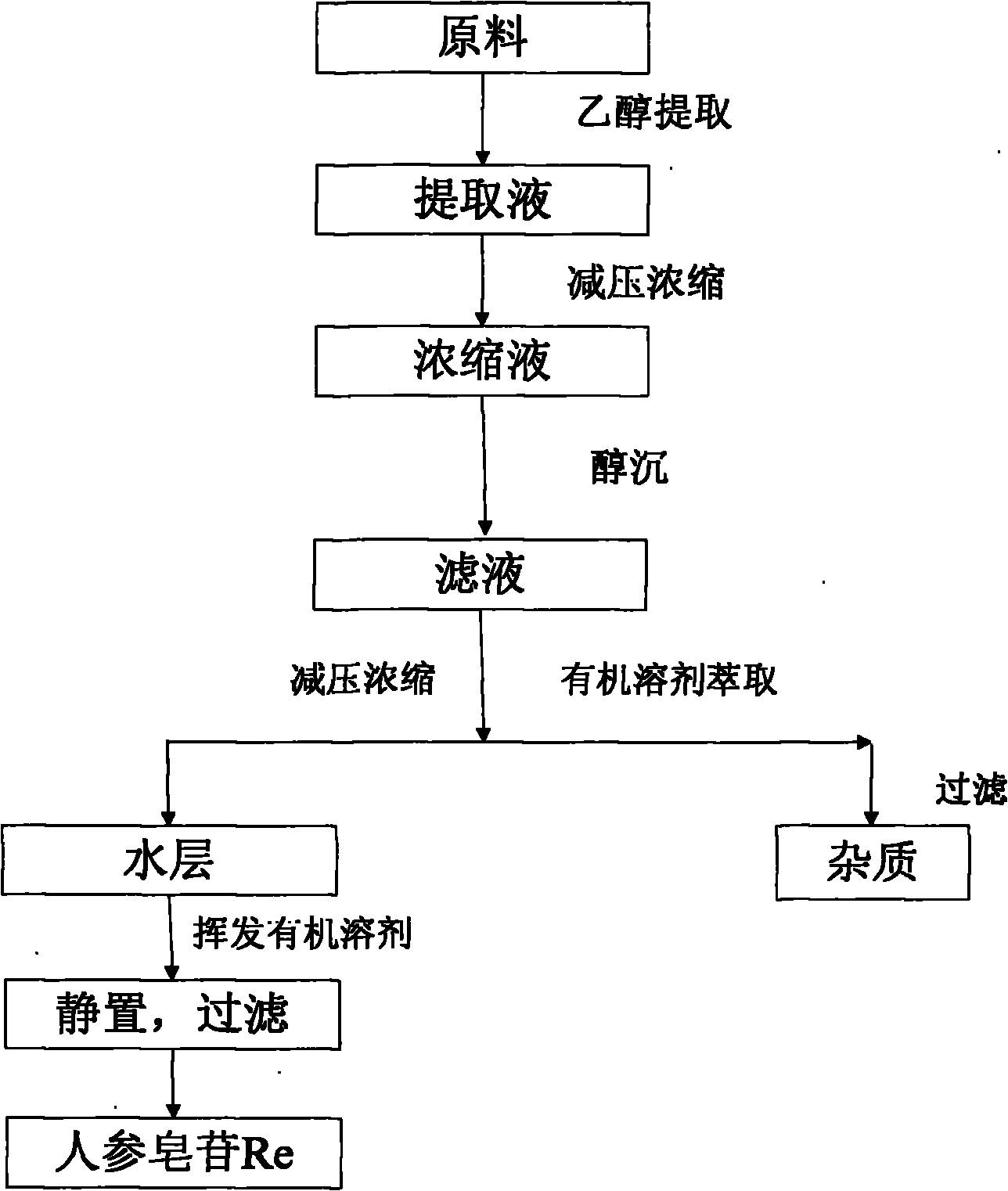
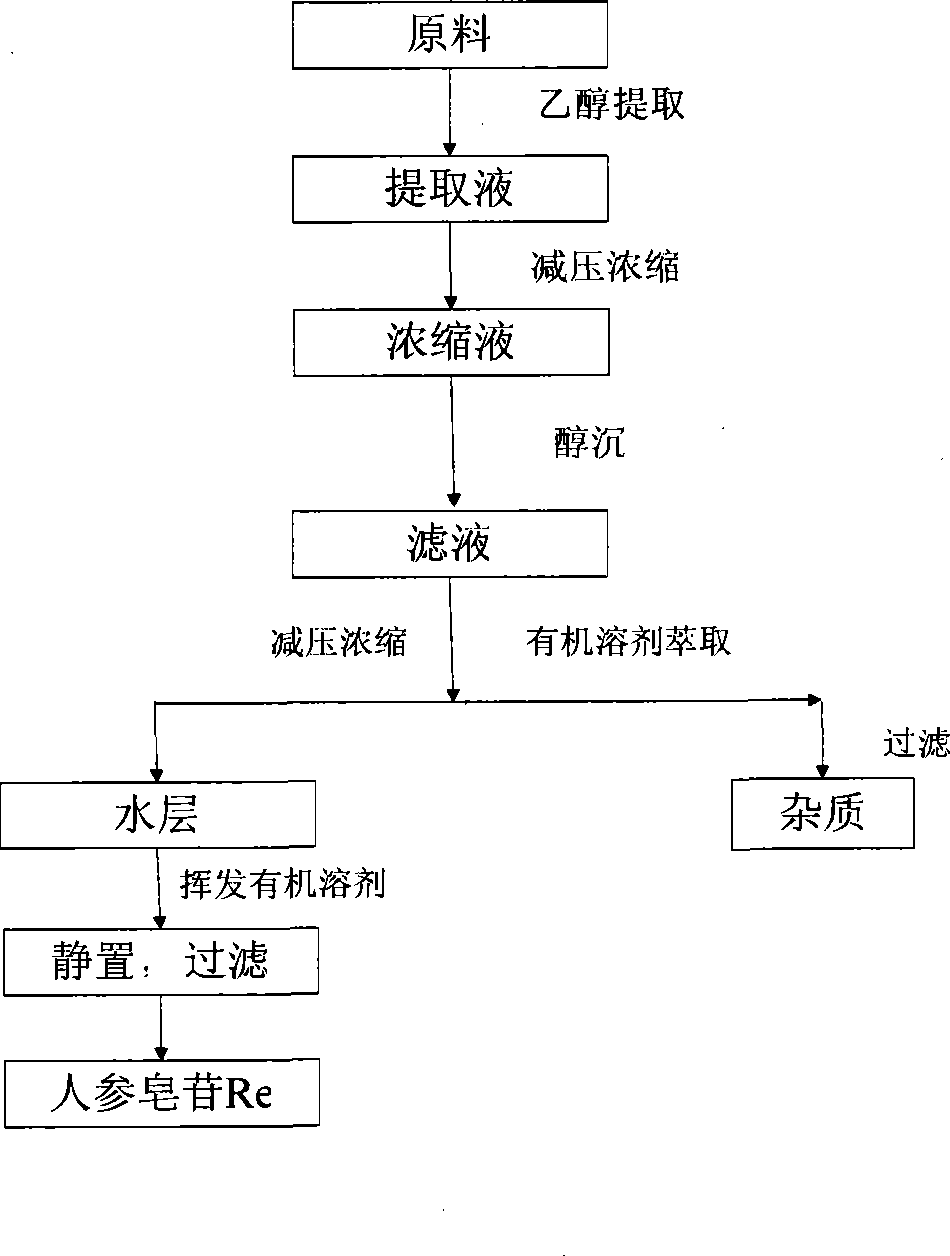
[0030] 1. Soaking and decoction of raw materials
[0031] Add the 60% ethanol solution into a steam distillation device at a weight ratio of raw material to solution of 1:15, soak at room temperature for 1 hour, heat to keep it in a slightly boiling state for 3 hours, filter while hot, collect the filtrate, and repeat extraction The operation is once, the extraction time is 0.5 hours, and the filtrates are combined.
[0032] 2. Concentration of extract
[0033] The filtrate obtained in step 1 was concentrated to 1 / 3 of the original extract volume under reduced pressure.
[0034] 3. Ethanol precipitation
[0035] Add 3 times the volume of ethanol solution to the concentrated solution obtained in step 2 for precipitation. The ethanol concentration is 30%. After standing for 24 hours, filter and collect the filtrate.
[0036] 4. Reconcentration of the extract
[0037] The filtrate obtained in step 3 is again concentrated under reduced pressure to 1 / 4 of the original volume.
[0038] 5. Organ...
Embodiment 2
[0043] Soaking and decoction of raw materials
[0044] Add the 90% ethanol solution into the steam distillation device at a weight ratio of raw material to solution of 1:5, soak at room temperature for 1 hour, heat to keep it in a slightly boiling state for 1 hour, filter while hot, collect the filtrate, and repeat extraction The operation was performed twice, the extraction time was 0.5 hours, and the filtrate was combined.
[0045] Concentration of extract
[0046] The filtrate obtained in step 1 was concentrated to 1 / 4 of the original extract volume under reduced pressure.
[0047] Ethanol precipitation
[0048] Add 3 times the volume of ethanol solution to the concentrated solution obtained in step 2 for precipitation. The ethanol concentration is 60%. After standing for 24 hours, filter and collect the filtrate.
[0049] Reconcentration of extract
[0050] The filtrate obtained in step 3 is again concentrated under reduced pressure to 1 / 4 of the original volume.
[0051] Organic solv...
Embodiment 3
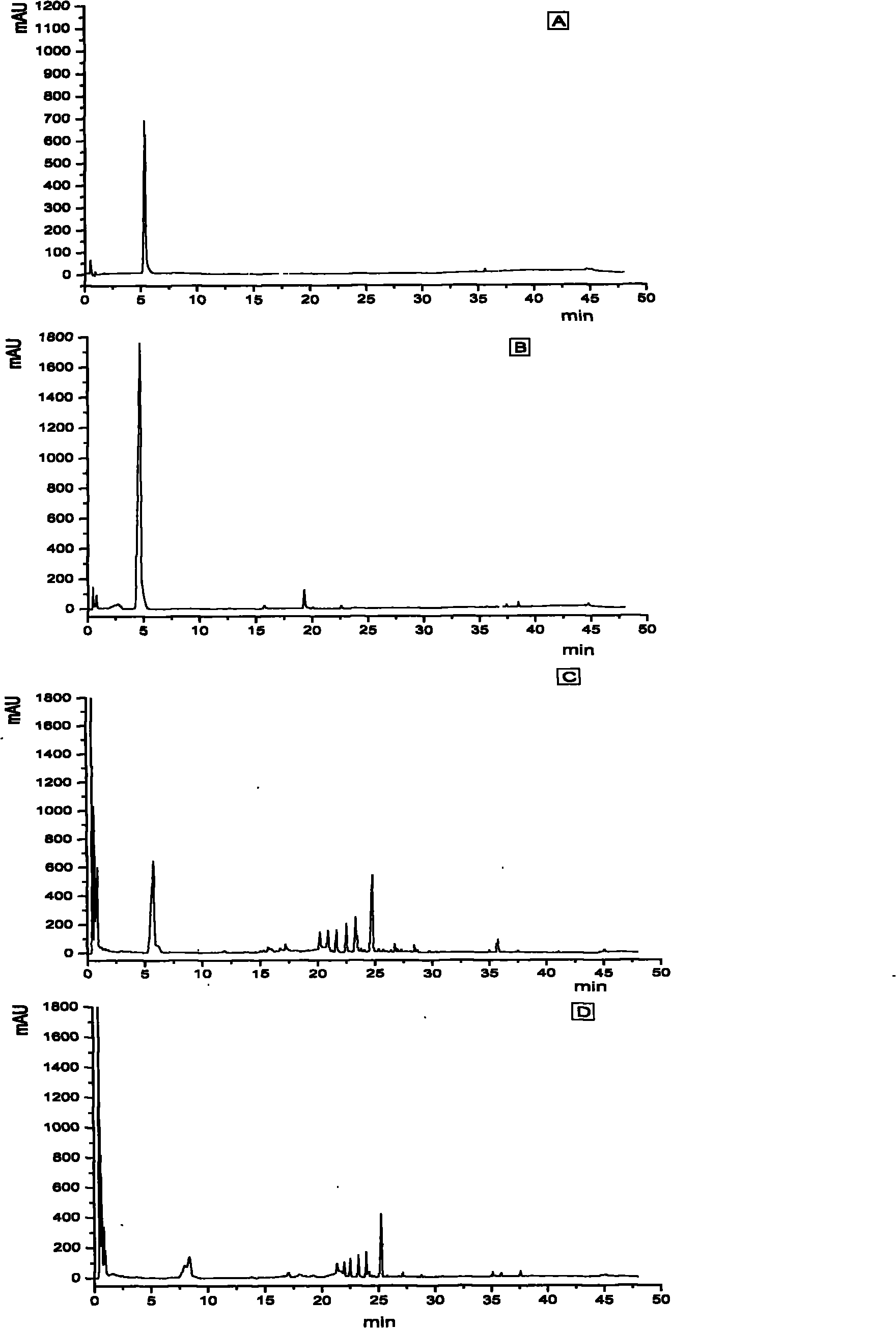
[0056] Sample liquid chromatography identification:
[0057] Apparatus and chromatographic conditions: high performance liquid chromatograph Agilent 1200SL, DAD detector, Chemstation workstation, ginsenoside Re reference substance purchased from China Pharmaceutical and Biological Products Laboratory (batch number: 110754-200822), chromatographic column: SDBC 18 1.8μm, 4.8×50mm, using acetonitrile-water as mobile phase, gradient elution, flow rate 1ml·min -1 , Detection wavelength is 203nm, column temperature is 25℃.
[0058] Test result: Calculated by normalization method with peak area as an index, the content of ginsenoside Re accounted for more than 80% of the total saponins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com