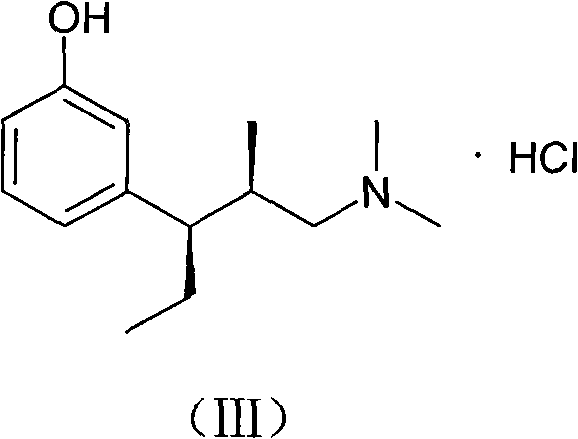
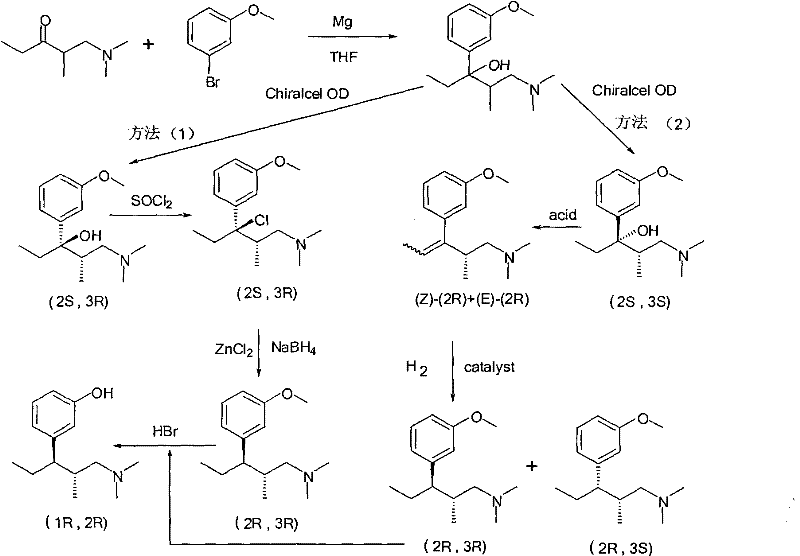
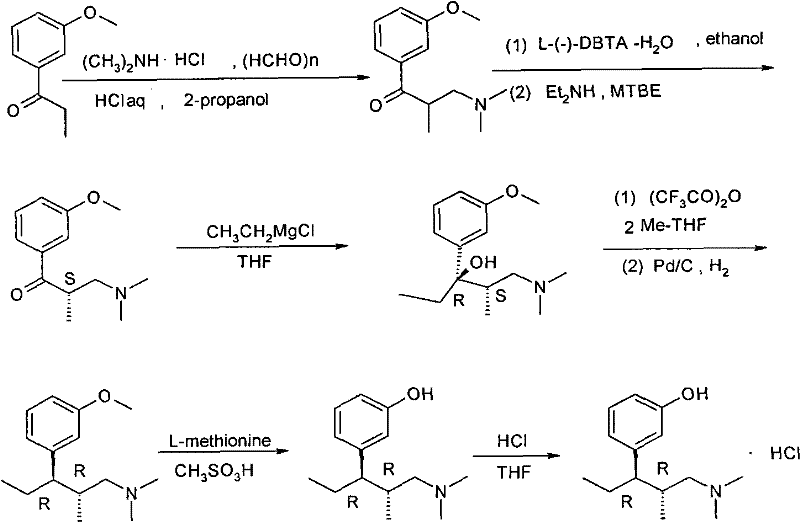
Synthesis and application of intermediate of tapentadol
A technology of tapentadol and intermediates, applied in the application field of preparing tapentadol and derivatives, can solve the problems of waste, long reaction time, R-configuration products can not be reused and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 (E, 4R)-3-(3'-m-methoxyphenyl)acryloyl-4-phenyl-2-oxazolidinone
[0087] Add 65g of m-methoxycinnamic acid to a 3L round bottom flask, dissolve it in 2L of tetrahydrofuran, add 94g of triethylamine at 0°C, then add 44g of pivaloyl chloride, stir the mixture for 1h, add 15.5g of lithium chloride, and divide into four times Add R-4-phenyl-2-oxazolidinone (13.5g×4), react at 0°C for 1h, at room temperature for 1h, filter with suction, concentrate, extract the residue with ethyl acetate / water and separate the layers, and the organic layer is washed with sulfuric acid The magnesium was dried, concentrated under reduced pressure, and then 600ml of diethyl ether was added, the temperature was raised slowly, stirred, and filtered to obtain 97g of white solid with a yield of 92%.
[0088] TOF-MS (m / z): 324 [M+1].
[0089] 1 H NMR (300M, CDCl 3 ): δ: 7.93(d, 1H, J=18.0Hz), 7.76(d, 1H, J=18.0Hz), 7.45-7.28(m, 6H), 7.20(d, 1H), 7.11(s, 1H) , 6.96(d, 1H), 5.57(dd, 1H),...
Embodiment 2
[0091] Example 2 (3'R, 4R)-3-(3'-m-methoxyphenyl)pentanoyl-4-phenyl-2-oxazolidinone
[0092] Put 2.46g magnesium bar in 100ml three-necked bottle, 20ml anhydrous tetrahydrofuran, under stirring slowly dropwise the anhydrous tetrahydrofuran solution (10.14g, 20ml) of ethyl bromide, keep reaction in reflux state, after ethyl bromide has been added, Heat to reflux for 1h.
[0093] Add 18.95g of ketone bromide-dimethyl sulfide complex and 100ml of anhydrous tetrahydrofuran into a 1L round bottom flask, cool down to -45°C, slowly add the Grignard reagent prepared above dropwise, react for 2 hours after the dropwise addition, and then Add (E,4R)-3-(3′-m-methoxyphenyl)acryloyl-4-phenyl-2-oxazolidinone in anhydrous tetrahydrofuran solution (20g, 200ml), react for 4h, and use 10 %NH 4 The reaction was terminated by Cl solution, extracted with ethyl acetate (50ml×3), the organic phases were combined, washed with water and saturated brine, dried over magnesium sulfate, concentrated und...
Embodiment 3
[0097] Example 3 (2'R, 3'R, 4R)-3-(2'-methyl-3'-m-methoxyphenyl)pentanoyl-4-phenyl-2-oxazolidinone
[0098] Add 13g (3'R, 4R)-3-(3'-m-methoxyphenyl)pentanoyl-4-phenyl-2-oxazolidinone to a 500ml round bottom flask, dissolve it with 100ml anhydrous tetrahydrofuran, Cool down to -45°C, slowly add 40ml of lithium bis(trimethylsilyl)amide (1M) dropwise, react for 2h, slowly add anhydrous tetrahydrofuran solution (5.48g, 50ml) of methyl iodide dropwise, react for 2h, heat up to - Reaction 2d at 20°C, add 10% NH 4 Cl solution, extracted with ethyl acetate (50ml×3), combined organic phases, washed with water, saturated brine, dried over magnesium sulfate, concentrated under reduced pressure, evaporated to dryness, and purified by column to obtain 10.8g of white solid with a yield of 80%.
[0099] TOF-MS (m / z): 368 [M+1].
[0100] 1 H NMR (300M, CDCl 3 ): δ: 7.33-7.18(m, 6H), 6.78(m, 3H), 4.88(dd, 1H), 4.23(m, 1H), 4.04(t, 1H), 3.98(dd, 1H), 3.81( s, 3H), 2.65(m, 1H), 1.89(m, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com