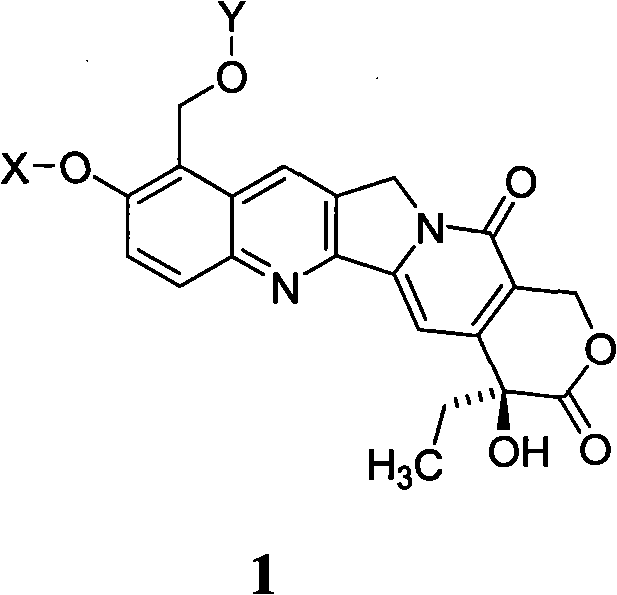
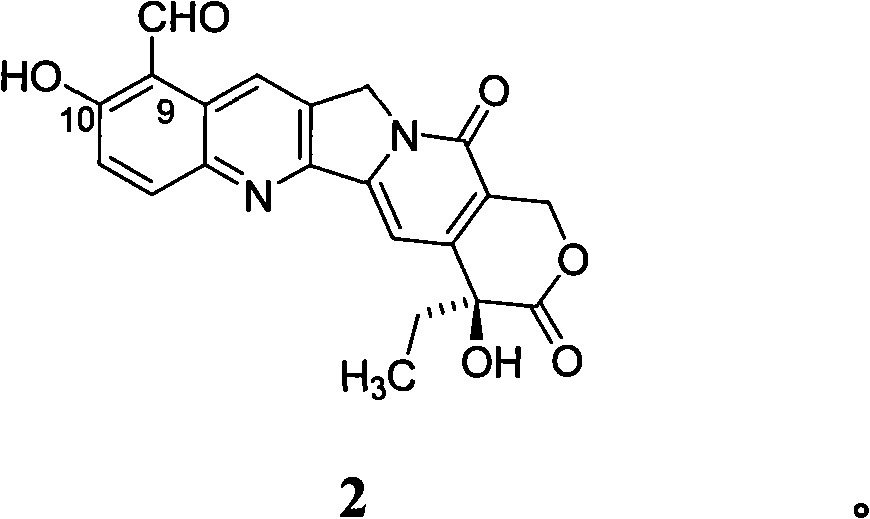
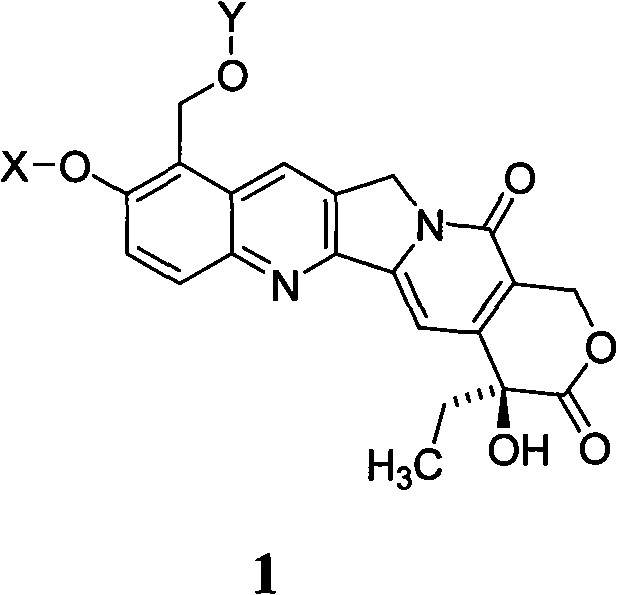
10-hydroxyamptothecin derivative, and its preparation method and application
A technology of hydroxycamptothecin and derivatives, applied in the field of medicine, can solve the problems of application limitation, poor solubility, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Synthesis of 9-formyl-10-acetoxycamptothecin
[0022]
[0023] Add 500mg (1.27mmol) of 9-formyl-10-hydroxycamptothecin and 100mL of anhydrous chloroform to a 250mL round bottom flask. After stirring, add 0.11mL (1.56mmol) of acetyl chloride and 0.36 of triethylamine. mL, heated and stirred at 50℃ to react, followed by TLC, and the reaction was complete after 1h. After the reaction solution cooled down, 100 mL of chloroform was added, washed successively with water, saturated sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, filtered to remove sodium sulfate, and concentrated under reduced pressure to obtain a yellow crude product, which was subjected to silica gel column chromatography. Elute with methyl chloride / methanol (60 / 1) to obtain 445.6 mg of pale yellow powder, with a molecular weight of 434.40 and a yield of 80.5%.
Embodiment 2
[0024] Example 2. Synthesis of 9-formyl-10-benzyloxycamptothecin
[0025]
[0026] In a 250mL round bottom flask, add 500mg (1.27mmol) of 9-formyl-10-hydroxycamptothecin, 526.6mg of anhydrous potassium carbonate, and 100mL of acetone. After stirring, add 0.45mL (3.79mmol) of benzyl bromide and heat. The reaction was stirred at reflux, followed by TLC, and the reaction was complete after 8 hours. After the reaction solution is cooled, it is filtered and the filter cake is washed with acetone. After the combined mother liquor is evaporated to remove the solvent under reduced pressure, it is recrystallized with acetone to obtain 548.4 mg of light yellow powder with a molecular weight of 482.48 and a yield of 89.5%.
Embodiment 3
[0027] Example 3. Synthesis of 9-hydroxymethyl-10-acetoxycamptothecin
[0028]
[0029] Add 500mg (1.15mmol) of 9-formyl-10-acetoxycamptothecin in a 50mL round bottom flask, and 25mL of methanol. After stirring to dissolve, add 65mg (1.72mmol) of sodium borohydride. Stir at room temperature for reaction, followed by TLC After 30 minutes, the reaction is complete. After filtration, the filtrate was evaporated to remove the solvent to obtain a crude product, which was recrystallized from chloroform / methanol to obtain 537.3 mg of pale yellow powder with a molecular weight of 436.41 and a yield of 85.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com