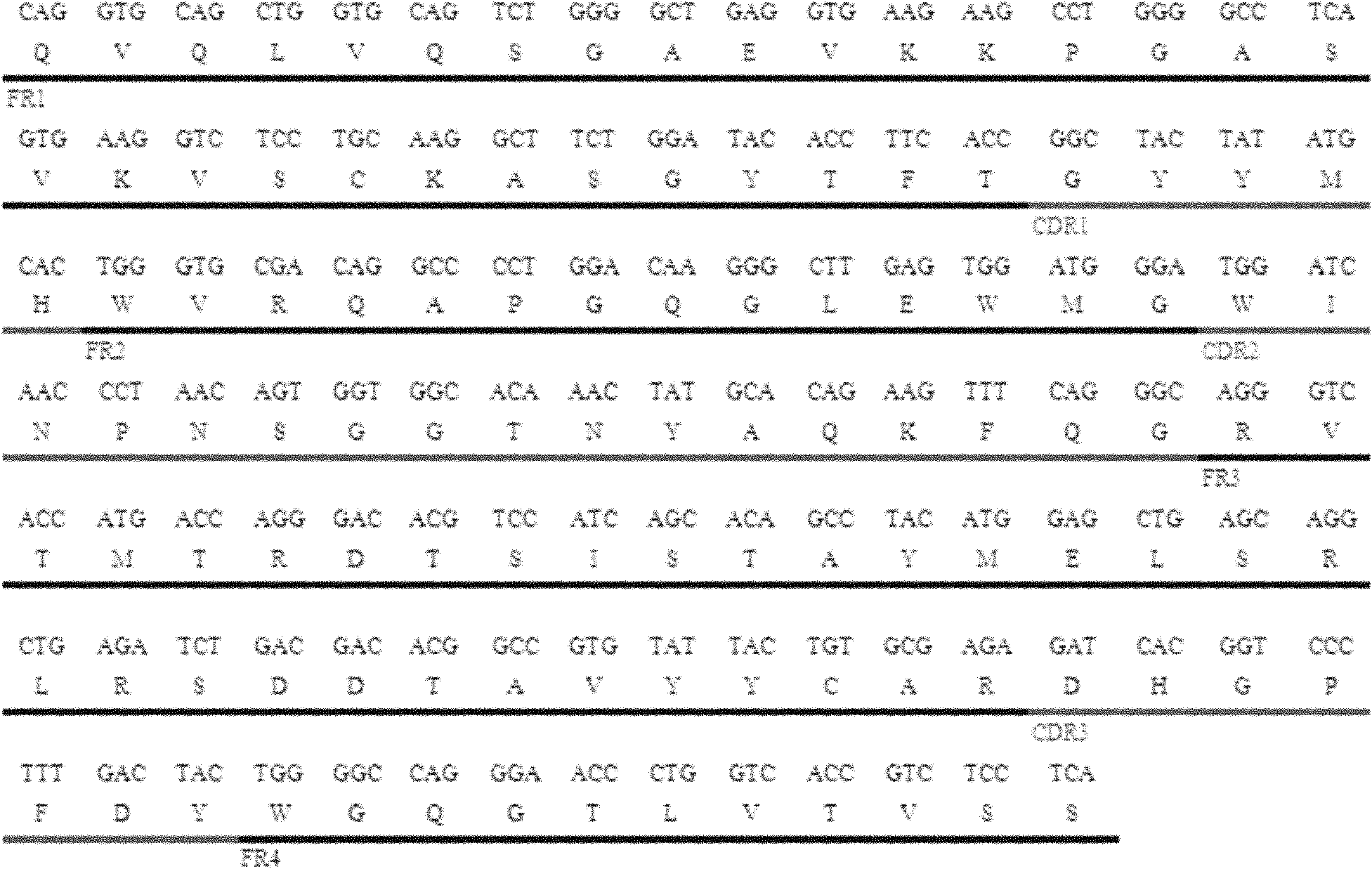Human derived heavy chain variable region possessing human vascular endothelial growth factor binding activity
A technology of vascular endothelium and composition, which is applied in the field of human heavy chain variable region and its coding sequence and application, and can solve the problems of lack of anti-VEGF antibody and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 Cloning of heavy chain variable region
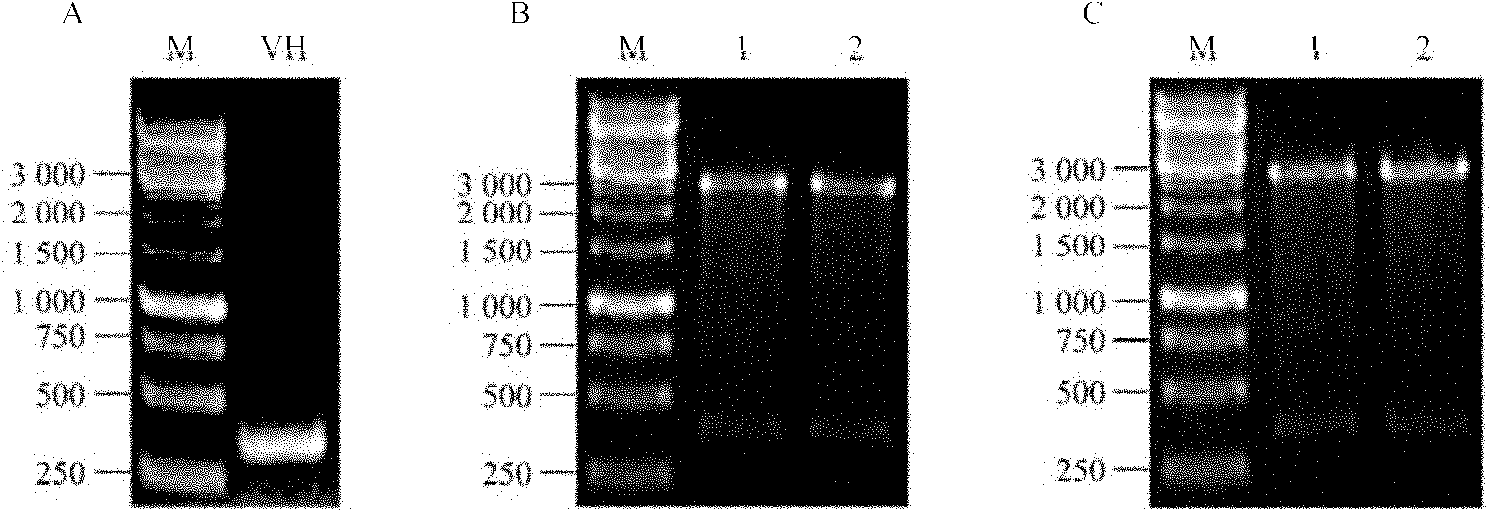
[0095] The total RNA extraction and reverse transcription of hybridoma cell V75 (CCTCC NO: C200623) were performed according to the kit instructions. Table 1 shows the PCR primers used for human immunoglobulin heavy chain variable region (VH) amplification. The PCR reaction conditions were: pre-denaturation at 94°C for 3 minutes; denaturation at 94°C for 30 seconds, annealing at 69°C for 30 seconds, extension at 72°C for 30 seconds, and 30 cycles; finally, extension at 72°C for 7 minutes. The PCR product of the expected size was recovered by 1.5% agarose gel electrophoresis and cloned into the pMD19-T vector.
[0096] Table 1 Universal primers used to amplify VH
[0097]
[0098] After primer pairing, it was determined that primer VH5-1 and primer VH3-3 paired can efficiently amplify a VH fragment of about 350bp ( figure 1 A). Insert the fragment into the pMD19-T vector to obtain the vector pMD19-T / VH, and the enzyme digest...
Embodiment 2
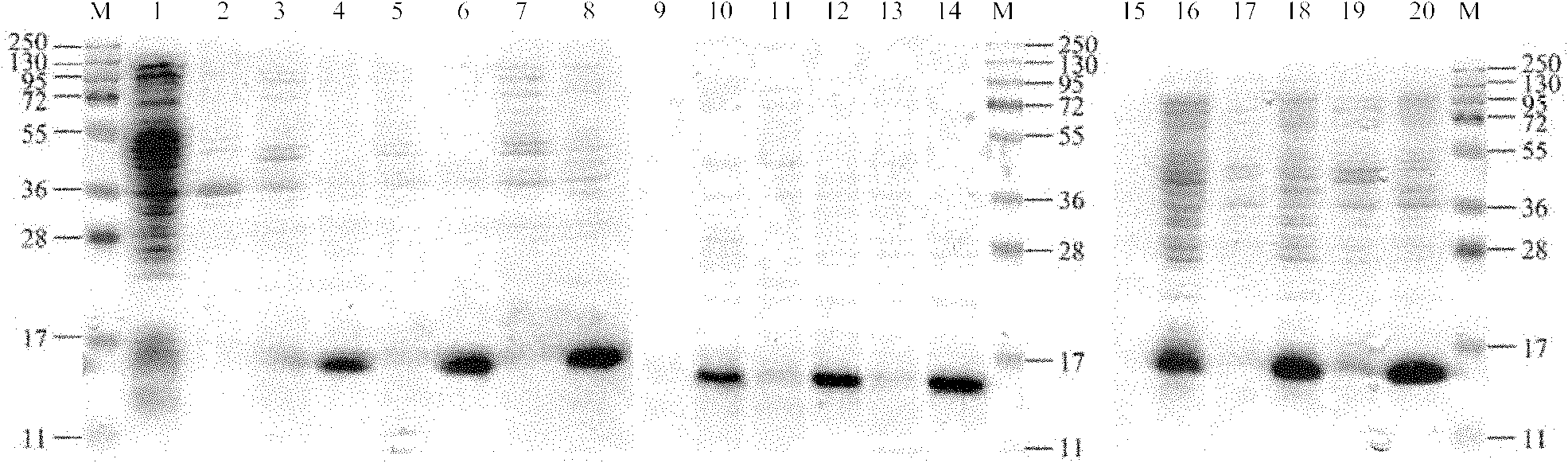
[0102] Example 2 Induced expression and purification of recombinant protein
[0103] Using the amplified product in Example 1 or the vector pMD19-T / VH as a template, use the VH-forward primer and VH-reverse primer shown in Table 1 (with Nde I and Hind III restriction sites, respectively) ) Perform PCR amplification. The amplified product (VH fragment) was digested with Hind III and Nde I, and ligated with the pET28a vector that was digested with Hind III and Nde I, so that the VH fragment was inserted into pET28a by Hind III and Nde I restriction sites. In the expression vector, the recombinant plasmid pET-VH is formed.
[0104] The pET-VH was transferred to the conventional E. coli BL21 recipient bacteria, and the optimal expression temperature (28℃, 32℃ and 37℃) and expression time (1h, 2h and 3h) were explored, and the rhVVH recombinant protein was expressed.
[0105] The amino acid sequence of the rhVVH recombinant protein is shown in SEQ ID NO: 11, in which positions 22-137 ar...
Embodiment 3
[0113] Example 3 Ability to bind antibodies to VEGF
[0114] The VEGF binding capacity of the recombinant rhVVH protein was carried out as follows: 0.5μg / mL human VEGF standard was coated with an ELISA plate, 100μL per well. The rhVVH was diluted in multiples, and VEGF monoclonal antibody (mAb produced by the V75 hybridoma cell line CCTCC NO: C200623, referred to as HVmAb, see Chinese patent application 200610116318.1)) and VEGF polyclonal antibody as a positive control group, pET28a empty The carrier extract served as a negative control group (NC). The dilution concentration and antibody used in each group are shown in Table 2, with 3 replicates in each group. Finally, the color was developed with OPD, the light absorption value of each sample at 490nm was measured, and the average value and variance were calculated.
[0115] Table 2 Sample dilution and antibody usage
[0116]
[0117] The results showed that although the primary antibody and enzyme-labeled antibody used in each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com