Application of integrin blocker polypeptide AP25 in preparation of medicines for treating tumor
An integrin blocker, AP25 technology, applied in the field of integrin blocker polypeptide AP25
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
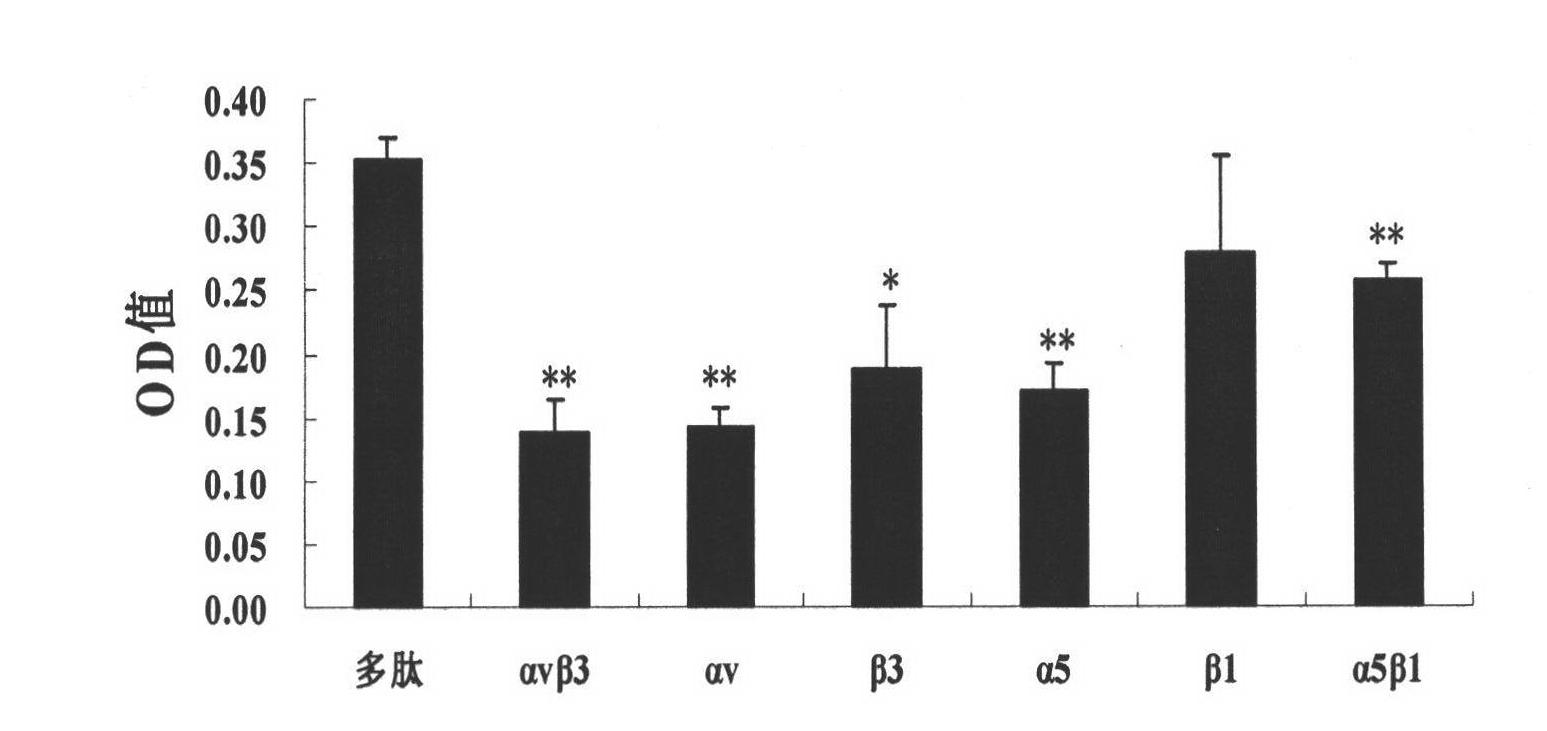
[0042] Integrin blocker peptide target analysis
[0043] The human umbilical vein endothelial cell (HUVEC) adhesion assay was used to determine the target of the integrin blocker polypeptide. That is, the 96-well plate was first spread with a certain concentration of polypeptide, and the HUVEC cells were incubated with different integrin subunit antibodies, and then the adhesion experiment was carried out. If cells incubated with an antibody show significantly less adhesion to the polypeptide than cells incubated without the antibody, it indicates that the polypeptide interacts with HUVEC through the integrin subunit. The specific operation is as follows: prepare the polypeptide into a 150 μg / ml solution with PBS, spread 100 μl per well on a 96-well plate, place at 4°C for 16 hours, and block with 1% BSA in a 22°C water bath for 4 to 5 hours. HUVEC cells (only use the 2nd to 5th passages) at 37°C, 5% CO 2 Incubate for 4 hours with serum-free endothelial cell culture medium w...
Embodiment 2
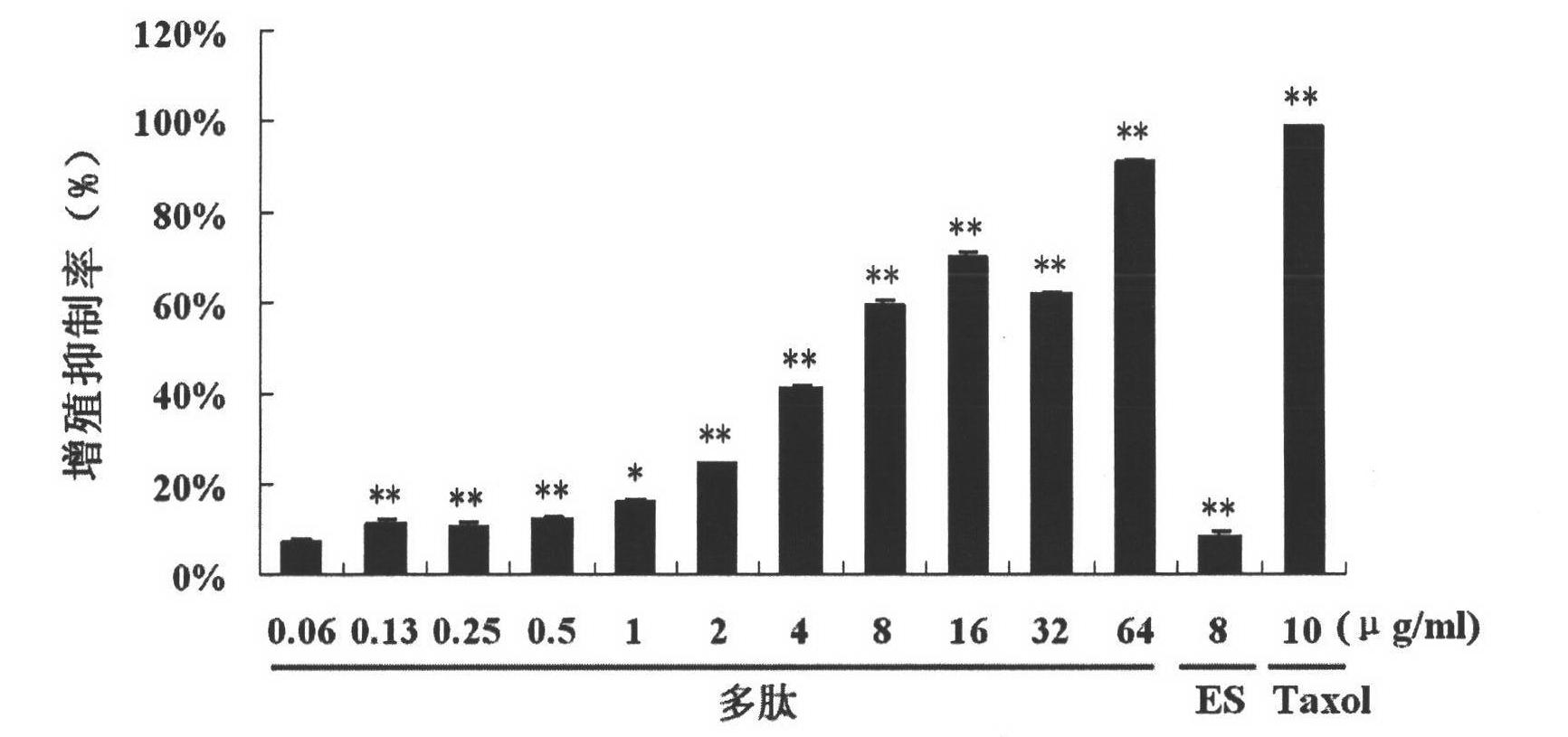
[0046] Proliferation Inhibitory Test of Integrin Blocker Peptides on Human Umbilical Vein Endothelial Cells (HUVEC)
[0047] The MTT method was used to detect the activity of the integrin blocker polypeptide in inhibiting the growth of endothelial cells, and the endothelial cells were only used in passages 2-6. HUVEC cells were incubated at 37°C, 5% CO 2 When the incubator was cultivated to a confluence of more than 90%, it was digested with trypsin and collected, and the cells were resuspended in the culture medium and counted under the microscope, and the cell concentration was adjusted to 3×10 4 cells / ml, inoculate the cell suspension into 96-well plate, 100 μl / well, and incubate at 37°C, 5% CO 2 Incubate overnight in the incubator. The integrin blocking agent polypeptide is diluted with culture medium to each predetermined concentration. Endostar was diluted with culture medium to the final concentration. After the cells were completely adhered to the wall, each diluti...
Embodiment 3
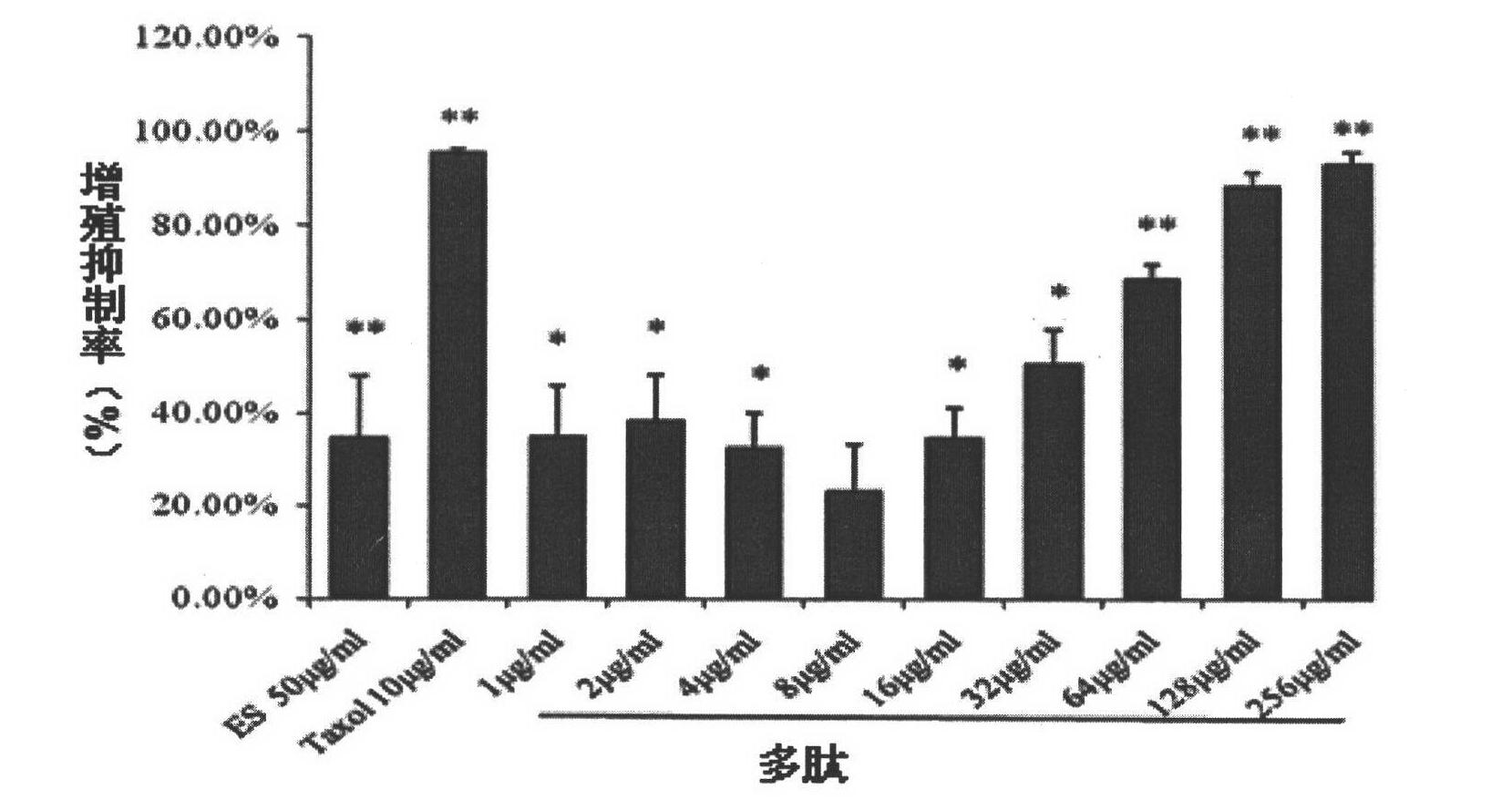
[0054] Proliferation Inhibition Test of Integrin Blocker Peptides on Human Gastric Cancer MGC-803 Cells
[0055] MTT method was used to detect the inhibitory activity of integrin blocker polypeptide on the growth of human gastric cancer MGC-803 cells. Tumor cells were incubated at 37°C, 5% CO 2 When the incubator was cultivated to a confluence of more than 90%, it was digested with trypsin and collected, and the cells were resuspended in the culture medium and counted under the microscope, and the cell concentration was adjusted to 2×10 4 cells / ml, inoculate the cell suspension into 96-well plate, 100 μl / well, and incubate at 37°C, 5% CO 2 Incubate overnight in the incubator. The integrin blocking agent polypeptide is diluted with culture medium to each predetermined concentration. Endostar was diluted with culture medium to the final concentration. After the cells were completely adhered to the wall, each dilution was added to a 96-well plate (100 μl / well). Adding the in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tumor inhibition rate | aaaaa | aaaaa |
| Tumor inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com