High molecular heavy metal chelating flocculant and preparation method thereof
A heavy metal and polymer technology, applied in chemical instruments and methods, flocculation/sedimentation water/sewage treatment, water/sewage treatment, etc., to achieve the effects of easy operation and control, high yield, and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
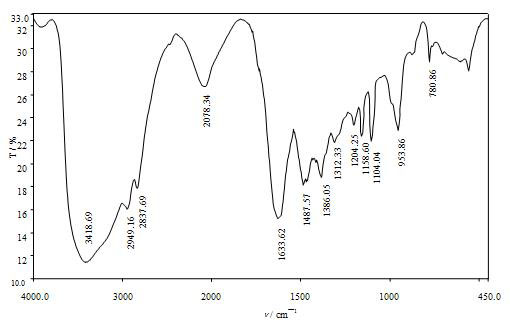
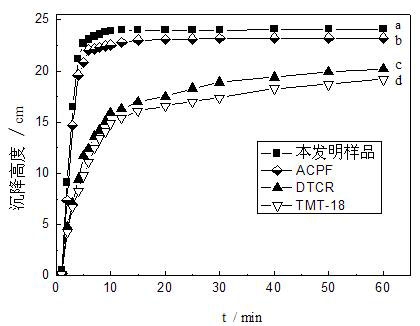
Image
Examples
Embodiment 1
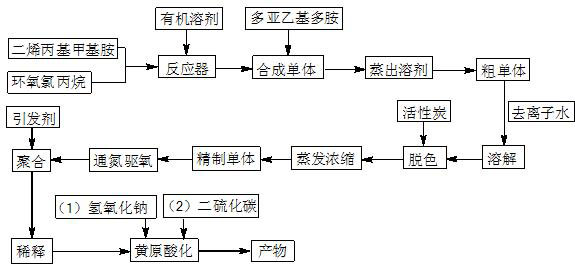
[0033] (1) According to the molar ratio of diallylmethylamine and epichlorohydrin as 1:1, respectively add 29.2mL diallylmethylamine and 15.8mL epichlorohydrin into the Add 300mL tetrahydrofuran to a 500mL three-neck flask with a reflux condenser and a mechanical stirrer, stir evenly, and heat up to 60°C for 10h;
[0034] (2) Add 21.9mL of diethylenetriamine dropwise, continue the reaction at 60°C for 6h, then distill off the solvent tetrahydrofuran to obtain 63.7g of crude monomer (pale yellow paste);
[0035] (3) Add 575mL of deionized water to dissolve the paste obtained in step (2) to form a solution with a mass percentage concentration of 10%, add activated carbon for decolorization, and then filter, and the filtrate is concentrated to a monomer mass percentage concentration by evaporation under reduced pressure Reach 60%, get refined monomer solution; Then pass N 2 Drive oxygen for 30 minutes, heat up to 60°C, add 712 mg of ammonium persulfate, react for 9 hours, and ob...
Embodiment 2
[0038] (1) According to the molar ratio of diallylmethylamine and epichlorohydrin as 1:1.02, respectively add 29.2mL of purified diallylmethylamine and 16.2mL of epichlorohydrin to the In the 1000mL three-neck flask with funnel, reflux condenser and mechanical stirrer, add 450mL tetrahydrofuran and stir evenly, heat up to 65°C and react for 9h;
[0039] (2) Add 29.9 mL of triethylenetetramine dropwise to the solution in step (1) and continue the reaction at 65°C for 5 hours, then distill off the solvent tetrahydrofuran to obtain 73.1 g of crude monomer (light yellow paste);
[0040] (3) Add 658mL of deionized water to dissolve the paste obtained in step (2) to form a solution with a mass percent concentration of 10%, add activated carbon for decolorization, and then filter, and the filtrate is concentrated to a monomer mass percent concentration by evaporation under reduced pressure Reach 65%, get refined monomer solution; Then pass N 2 Drive oxygen for 30 minutes, raise the ...
Embodiment 3
[0043] (1) According to the molar ratio of diallylmethylamine and epichlorohydrin as 1:1.05, respectively add 29.2mL of purified diallylmethylamine and 16.7mL of epichlorohydrin to the Into a 1000mL three-necked flask with a funnel, a reflux condenser and a mechanical stirrer, add 600mL of tetrahydrofuran, stir evenly, and heat up to 70°C for 9 hours;
[0044] (2) Add 38.4 mL of tetraethylenepentamine dropwise to the solution in step (1) and continue the reaction at 70°C for 5 hours, then distill off the solvent tetrahydrofuran to obtain 81.2 g of crude monomer (pale yellow paste);
[0045] (3) Add 730mL of deionized water to dissolve the paste obtained in step (2) to form a solution with a mass percent concentration of 10%, add activated carbon for decolorization, and then filter, and the filtrate is concentrated to a monomer mass percent concentration by evaporation under reduced pressure Reach 70%, get refined monomer solution; Then pass N 2 Drive oxygen for 30 minutes, ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com