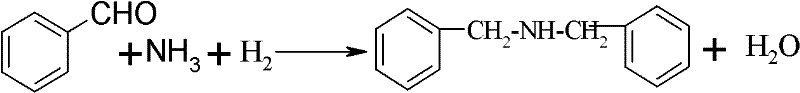
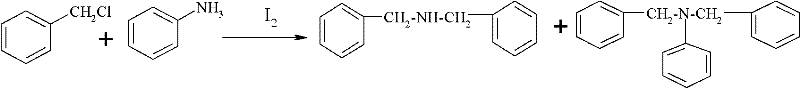Preparation method of dibenzylamine
A technology of dibenzylamine and benzaldehyde, which is applied in the field of preparation of dibenzylamine, can solve the problem of low yield of dibenzylamine, and achieve the effects of reduced production cost, extended service life and improved conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 1000mL autoclave, add 200g of benzaldehyde, 45g of ammonia, 100g of pure water as a solvent, 5g of a catalyst, the content of the catalyst is 0.3% of Pd, 0.1% of Sn, and the rest is the carrier TiO 2 . The reaction temperature is 60° C., the hydrogen reaction pressure is 2.0 MPa by gauge pressure, the reaction time is 20 minutes, the conversion rate of benzaldehyde is 100%, and the yield of dibenzylamine is 97.2%.
[0020] The catalyst wherein is prepared as follows: soak the carrier in a 30% nitric acid solution, reflux at 100°C for 7 hours, wash to neutrality, add required amount of palladium chloride and sodium hydroxide solution in the aqueous solution of the carrier , and adding Sn as a cocatalyst for modification, and TiO 2 As a carrier, it is prepared after washing with water and drying.
Embodiment 2~5
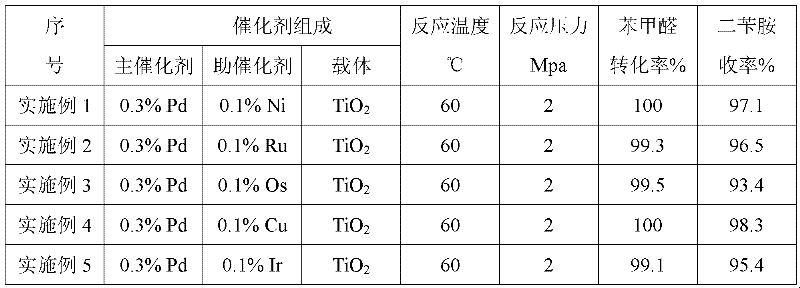
[0022] According to each operation step and condition of embodiment 1, only change the composition of catalyst, its reaction result is listed in table 1.
[0023] Table 1
[0024]
Embodiment 6~10
[0026] According to each operation step and condition of embodiment 1, adopt the catalyst used among the embodiment 4, its consumption is 2.5% of benzaldehyde, solvent consumption is 50% of benzaldehyde, change reaction condition, its reaction result is listed in table 2.
[0027]
[0028] As can be seen from the data in Table 1 and Table 2, under the process conditions, benzaldehyde has a higher conversion rate and selectivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com