Cation light-cured alicyclic epoxy compound and preparation method thereof
A cycloaliphatic epoxy and compound technology, which is applied in the field of cationic photocuring and photocuring, can solve the problem of single variety of cycloaliphatic epoxy compounds, and achieve the effect of various types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of Cationic Photocurable Cycloaliphatic Epoxy Compound Based on 1,6-Hexanediol Diacrylate (HDDA):
[0027] Add 169.5 g of 1,6-hexanediol diacrylate (0.75 mol) and 99.2 g of cyclopentadiene (1.5 mol) into a 500 mL three-necked flask equipped with a reflux condenser, drying tube and mechanical stirring, and stir at room temperature After 72 hours, the intermediate HDN can be obtained through its addition reaction;
[0028] Add 60.8 g (0.30 mol) of m-chloroperoxybenzoic acid and 600 mL of dichloromethane into a 1000 ml three-neck flask equipped with a reflux condenser, a drying tube, and a magnetic stirrer, cool to 0°C in an ice-salt bath, and slowly add Add dropwise a mixed solution of 35.8g (0.100 mol) of intermediate HDN and 100 mL of dichloromethane for 2 hours. After the dropwise addition, keep stirring at room temperature for 24 hours, then cool the mixture to below 0°C, and filter the precipitated White crystals, the filtrate was washed with ...
Embodiment 2
[0030] Example 2: Preparation of Cationic Photocurable Alicyclic Epoxy Compound Based on Trimethylolpropane Triacrylate (TMPTA):
[0031] Add 148.1 g of trimethylolpropane triacrylate (0.50 mol) and 99.2 g of cyclopentadiene (1.5 mol) into a 500 mL three-necked flask equipped with a reflux condenser, drying tube and mechanical stirring, and stir at room temperature for 72 hours , through its addition reaction, the intermediate TAN can be obtained;
[0032] Add 41.7 g (0.24 mol) m-chloroperoxybenzoic acid and 500 mL butyl acetate into a 1000 ml three-neck flask equipped with a reflux condenser, a drying tube and a magnetic stirrer, cool to 0°C in an ice-salt bath, and slowly add Add dropwise a mixed solution of 33.1g (0.067 mol) intermediate TAN and 100 mL of dichloromethane for 3 hours. After the dropwise addition, keep stirring at room temperature for 24 hours, then cool the mixture to below 0°C, and filter the precipitated White crystals, the filtrate was washed with 20% so...
Embodiment 3
[0034] Embodiment 3: Preparation of cationic photocurable cycloaliphatic epoxy compound based on acrylate compound TAEP:
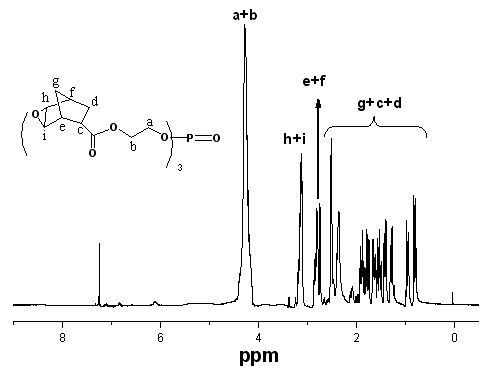
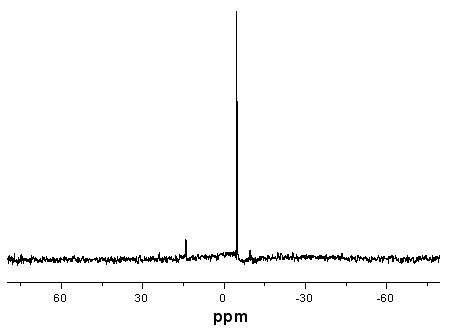
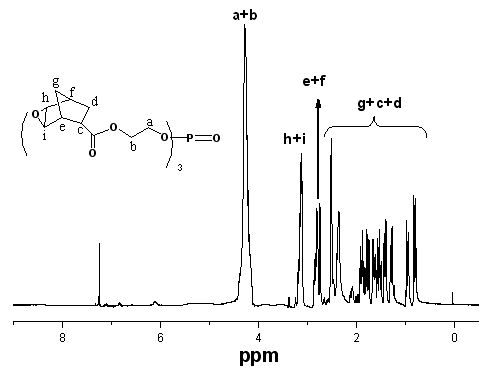
[0035] Add 100 mL of dichloromethane, 19.2 g of TAEP (0.05 mol) and 11.9 g of cyclopentadiene (0.18 mol) into a 250 mL three-necked flask equipped with a reflux condenser, drying tube and mechanical stirring, and stir the reaction at room temperature for 72 h, Distilled under reduced pressure to obtain a colorless liquid intermediate TNP.
[0036] Add 41.7 g (0.24 mol) of m-chloroperoxybenzoic acid and 500 mL of ethyl acetate into a 1000 ml three-neck flask equipped with a reflux condenser, a drying tube, and a magnetic stirrer, cool to 0°C in an ice-salt bath, and slowly add Add dropwise a mixed solution of 39.5g (0.067 mol) intermediate TNP and 100 mL of dichloromethane for 2.5 hours. After the dropwise addition, keep stirring at room temperature for 24 hours, then cool the mixture to below 0°C and filter out white crystals, the filtrate was washed with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com