Synthetic technology of diphenylmethane dicarbamate by adopting Bronsted-Lewis acidic ionic liquid catalysis
A technology of methyl dicarbamate and methyl phenylcarbamate, which is applied in the field of diphenylmethane dicarbamate synthesis technology catalyzed by Br*nsted-Lewis double-acidic ionic liquid, can solve the problem that the catalyst is difficult to be reused and the product Low yield and other problems, to achieve the effect of easy preparation and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
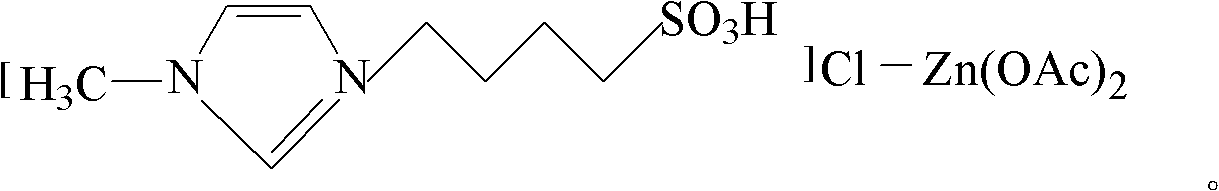
[0033] used in the present invention The preparation method of bis-acidic ionic liquid:
[0034] With reference to the technical route provided by Liu et al. (Catalysis Communications, 2008, 9: 2030-2034), the cations can be synthesized as 1-(4-sulfonic acid)butyltriethylammonium, 1-(3-sulfonic acid)propyltriethylammonium Ammonium butyl ammonium, 1-(4-sulfonic acid) butyl tripropyl ammonium, 1-(3-sulfonic acid) propyl tripropyl ammonium, 1-(4-sulfonic acid) butyl tributyl ammonium and 1- (3-sulfonic acid)propyltributylammonium, anion is AlCl 4 - , ZnCl 3 - , FeCl 4 - , SnCl 3 - and CuCl 2 - of Biacidic ionic liquid; reference to the method provided in Liu et al. 1-(3-sulfonic acid)propyl-3-ethylimidazole, 1-(4-sulfonic acid)butyl-3-methylimidazole, 1-(3-sulfonic acid)propyl-3-methylimidazole , 1-(3-sulfonic acid) propylpyridine and 1-(4-sulfonic acid) butylpyridine, the anion is AlCl 4 - , ZnCl 3 - , FeCl 4 - , SnCl 3 - and CuCl 2 - of Biacidic ionic...
Embodiment 1
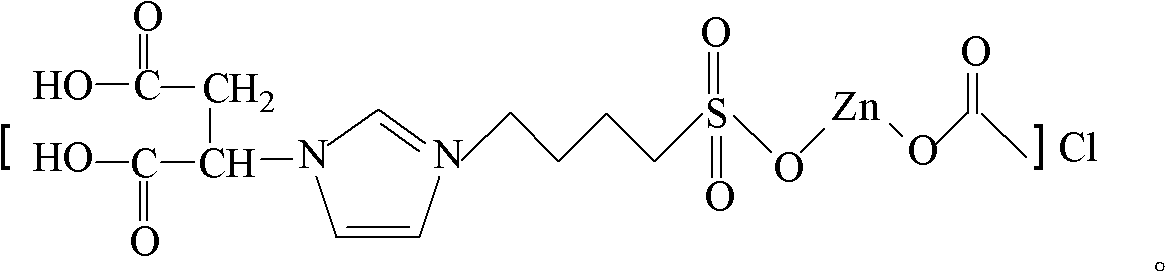
[0036] Dissolve 1 mol of imidazole in 150 ml of acetone, add 1.1 mol of diethyl maleate, react at reflux temperature for 24 hours, distill under reduced pressure, distill off the solvent and unreacted raw materials to obtain 1-(2-butanedioic acid diethyl ester group) imidazole, then at 40°C, 1-(2-succinate diethyl) imidazole and 1,4-butane sultone were mixed in a molar ratio of 1:1.1, reacted for 20h, washed with ether, After drying, 1-(2-diethylsuccinate)-3-(4-sulfonate)butylimidazolium zwitterion was obtained. Take 0.5mol of the above-mentioned zwitterions and dissolve them in 100ml of water, add 3mol of concentrated hydrochloric acid (36.5% in mass concentration) dropwise, react at 80°C for 6h, and distill under reduced pressure (vacuum degree 0.01MPa, the following steps and examples are the same as the vacuum degree ) to remove water and unreacted hydrochloric acid to obtain 1-(2-butanedioic acid)-3-(4-sulfonic acid) butylimidazole chloride salt, then get 0.22mol zinc ace...
Embodiment 2
[0044] Dissolve 1 mol of imidazole in 150 ml of acetone, add 1 mol of diethyl maleate, react at reflux temperature for 12 hours, after vacuum distillation, distill off the solvent and unreacted raw materials to obtain 1-(2-butanedioic acid diethyl ester group) imidazole, then at 80°C, 1-(2-succinate diethyl) imidazole and 1,4-butane sultone were mixed in a molar ratio of 1:1, reacted for 10h, washed with ether, After drying, 1-(2-diethylsuccinate)-3-(4-sulfonate)butylimidazolium zwitterion was obtained. Take 0.5 mol of the above-mentioned zwitterions and dissolve them in 100 ml of water, add 1.5 mol of concentrated hydrochloric acid (36.5% in mass concentration) dropwise, react at 130°C for 1 h, and distill off water and unreacted hydrochloric acid under reduced pressure to obtain 1-(2- Succinic acid)-3-(4-sulfonic acid) butylimidazole chloride, then take 0.2mol of zinc acetate and dissolve it in 100ml of water, add 0.2mol of 1-(2-butanedioic acid)-3-(4-sulfonic acid ) butyli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com